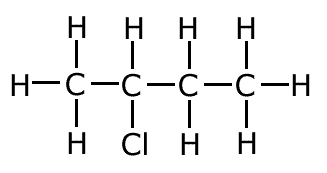|
Due to the many millions of different structural arrangements of organic compounds, a rigorous naming system was needed to avoid ambiguity. This naming system is called organic nomenclature. |
Systematic nomenclature
There are millions of different organic molecules. To give every one of them a different name would involve a huge amount of learning, were no logical pattern to be adopted.
The IUPAC developed the systematic naming system (nomenclature) to allow many of the organic compounds to be named in a logical fashion by considering the structural features of the molecule. This systematic nomenclature arrives at the compound name in the following order:
- Step 1 - Longest carbon chain
- Step 2 - Unsaturation
- Step 3 - Functional groups
- Step 4 - Position of any functional groups
Step 1: Find the longest unbroken carbon chain and use this as the basis for the root name of the compound.
|
Number of carbon atoms
|
Root
|
|
1
|
Meth-
|
|
2
|
Eth-
|
|
3
|
Prop-
|
|
4
|
But-
|
|
5
|
Pent-
|
|
6
|
Hex-
|
Step 2: The term 'saturation' and unsaturation' refer to the types of bonds that make up the carbon skeleton. Double and triple bonds are said to be 'unsaturated', whereas a molecule that contains only single carbon-carbon bonds is said to be 'saturated'.
- Unsaturated hydrocarbon chains containing a double bond have the ending modified to -ene.
- Unsaturated hydrocarbon chains containing a triple bond have the ending modified to -yne.
Rule 3: Look for any different atoms or groups of atoms attached to the longest carbon chain. (an atom that is not carbon or hydrogen is called a heteroatom)
|
Group of atoms
|
suffix
|
|
C=C
|
-ene
|
|
CC
|
-yne
|
|
-OH
|
-anol
|
|
-CHO
|
-anal
|
|
-COOH
|
-anoic acid
|
|
-NH2
|
-ylamine*
|
Some heteroatoms or groups of atoms are tagged onto the front of the root like a prefix. The following table shows groups that appear as a prefix to the root:
|
Atom or group of atoms
|
prefix
|
|
-Cl
|
chloro
|
|
-Br
|
bromo
|
|
-I
|
iodo
|
|
-NO2
|
nitro
|
|
-NH2
|
amino*
|
|
CnH(2n+1)
|
alkyl**
|
Note 1: * The NH2 group appears in both lists. Both systems of naming amines are still used.
Note 2: ** The term 'alkyl' refers to any alkane chain that has one fewer hydrogen atom, see branching below:
If there are two carbon chains joined by a functional group, then each must be named separately. This is the case with ethers, esters and amides.
Rule 4: Use of locants: The carbon chain is now examined for attachments such as other alkyl groups, halogen atoms etc. If there are attachments then the carbon chain is numbered from one end so that the attachments fall on the carbon with the lowest number. These attachments are then named as a prefix, using the numbered position of the attachment on the carbon chain to indicate their position.
|
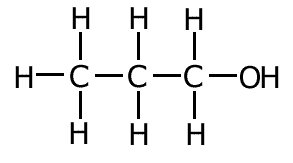
Example: What is the correct systematic name for the following molecule?:
Therefore the systematic name = propan-1-ol Although this is the correct systematic name for the molecule, you may also see it described as 1-propanol. This is considered acceptable. |
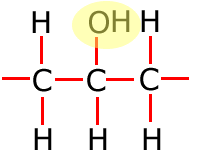
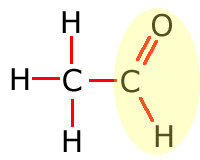
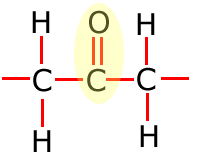
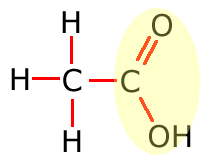
Functional groups
Functional groups are considered to be any atom or groups of atoms that does not consist of carbon, hydrogen and single bonds only.
In the table above, they have been shown in shorthand (condensed) form. This doesn't explain clearly how they are bonded onto the main carbon chain and in the following sections the structures of the functional groups are dealt with one by one. In brief, however, the structures of the functional groups are shown highlighted below:
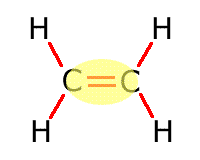 |
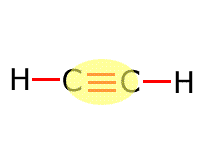 |
 |
|
Alkene
|
Alkyne
|
Alcohol
|
|
|
||
 |
 |
 |
|
Aldehyde
|
Ketone
|
Carboxylic acid
|
Branching
The main carbon chain may have other carbon chains attached. This is called branching and the smaller carbon chains are referred to as the branches. Each of the branched chains must be named according to the number of carbons that it contains and, if there is a possibility of ambiguity, the position of the branch on the main carbon chain must be shown as a number (locant).
|
Number of carbon atoms in the
branch
|
Prefix-
|
|
1
|
Methyl
|
|
2
|
Ethyl
|
|
3
|
Propyl
|
|
4
|
Butyl-
|
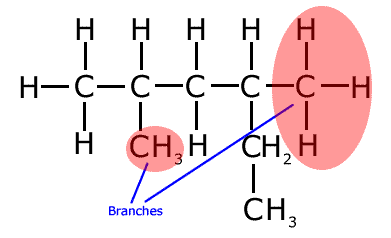
Care must be taken to identify the longest chain. It is important not to be put off by chains that change direction - this is NOT a branch! Take a look at the following molecule and make sure that you know why the two areas under red shadow are the real branches in this molecule.
The longest chain in the molecule above has six carbon atoms, hence the root name is 'hex-' (Run your mouse over the structure to see the longest unbroken chain).
Even though it seems that there is a two carbon branch on carbon number 4, in reality the longest chain runs left to right for four carbons and then down for two, it has six carbon atoms. That means that the other hydrocarbon groups are branches. The correct name (numbering left to right) of the molecule is 2,4-dimethylhexane.
This type of problem represents a favorite question for examiners, who try to conceal the longest chain by bending it and changing direction. Remember that a branch is literally that; a carbon atom junction from which there are two choices of two carbon atoms to extend the chain.
|
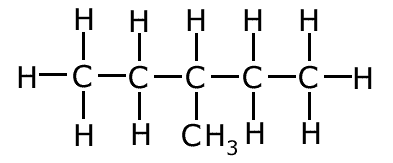
Example: Name the following molecule:
The main carbon chain has five carbon atoms, so the root of the name is pent- There are no atoms other than carbon and hydrogen and no multiple bonds, so the suffix '-ane' must be added to the root, giving pentane There is one branch on the chain containing one carbon atom = 'methyl-' . This is placed before the root as a prefix, giving methylpentane. However there are two places that the branch could be, on carbons number 2 and 3. In this case the branch is on carbon number 3, so this must be added to the name. Therefore the systematic name is 3-methylpentane Question Why couldn't the branch be on carbon number 1 or 5? Because this would add one carbon atom to the main chain making the root hex- instead of pent-. Question Why is 4-methylpentane an impossible name? The rules state that the carbon chain must be numbered in such a way as to keep the numbers in the name as low as possible. If the attachment were to be on carbon number 4, this would also be carbon number 2, numbering from the other end of the chain. |
Multiple attached groups
In the event that there is more than one group of the same type attached to the carbon chain, this is indicated in the naming system by using a special prefix in front of the group name (a multiplier). For example, if we wish to say that there are two of a specific group, then the prefix 'di-' is used. Once again the positions of the groups must be indicated if any ambiguity is possible.
|
groups the same
|
prefix
|
|
two
|
di-
|
|
three
|
tri-
|
|
four
|
tetra-
|
|
five
|
penta-
|
|
six
|
hexa-
|
Hence, a molecule that contains four chlorine atoms will have the prefix 'tetrachloro-', three methyl groups 'trimethyl-', etc.
|
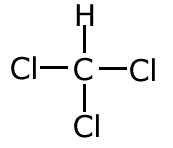
Example: Use the systematic nomenclature to name the following molecule -
There is only one carbon in the longest chain, so the name is based on meth-. There are no functional groups to affect the ending, so the molecule has the root methane. There are three chlorine atoms attached, hence trichloro- Therefore the systematic name is trichloromethane |
Primary, secondary and tertiary
These terms are used to describe the environment of atoms within a chain.
For example, a primary carbon atom has only one other carbon atom attached to it. A secondary carbon atom has two other carbon atoms attached to it and a tertiary carbon atom has three other carbon atoms attached.
The terminology is not restricted to carbon atoms, but can also be applied to alcohols and haloalkanes. In these cases, it is necessary to understand that it is the carbon atoms that the functional atom, or group, is attached to that is primary secondary or tertiary.
|
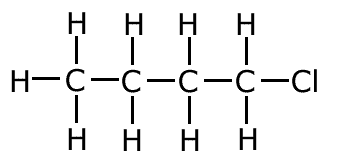
2-chlorobutane (secondary haloalkane)
|
1-chlorobutane (primary haloalkane)
|
The following abbreviations are used to indicate the type of carbon associated with the functional group:
- Primary = 1º
- Secondary = 2º
- Tertiary = 3º
Arenes
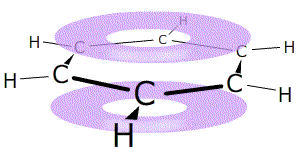 The term 'aromatic' refers to compounds that contain benzene rings. These
are also called arenes.
The term 'aromatic' refers to compounds that contain benzene rings. These
are also called arenes.
The structure of benzene, C6H6, was discussed in section 2.28
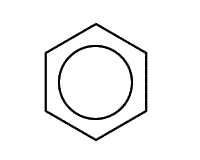
A benzene ring consists of a hexagon of six carbon atoms in a closed circle, each carbon connected to one hydrogen atom. The rings are drawn using a shorthand of a hexagon with a circle that represents the delocalised electrons inside.
Remember that each point of the hexagon represents one carbon atom and one hydrogen atom, unless there is a substituting group.
 |
 |
 |
|
Benzene
|
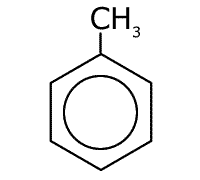
Methylbenzene
|
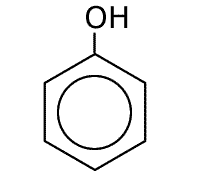
Phenol
|
Naming benzene compounds is semi-systematic, in that the ring carbons are numbered from 1 to 6, starting with any attachment to the ring, and these carbons are not counted as part of any carbon chain. This applies to benzene rings with two or more attachments.
Nomenclature in benzene compounds
Nitrogen containing compounds
Nitrogen is a very important element in organic chemistry. It is found in
the majority of compounds involved in living systems. 
Proteins and the DNA of plant and animal cells are made up of nitrogen containing compounds.
Nitrogen is sometimes called a heteroatom, one that is not carbon, hydrogen or oxygen. Simple compounds containing nitrogen include amines, amides, and nitriles.
Amines
Amines contain nitrogen atoms attached to either alkyl groups or hydrogen and alkyl groups. It can be considered as an ammonia molecule in which one, or more, of the hydrogen atoms has been replaced by an alkyl group.
The basic structure of an amine is R-NH2 (where 'R' represents an alkyl chain). The alkyl group can occupy either one, two or three sites on the nitrogen giving rise to primary, secondary and tertiary amines.
The names of amines come from the longest alkyl chain name plus -amine as a suffix.
| Structures and names of primary amines | ||
|---|---|---|
 |
 |
 |
|
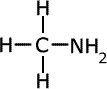
methylamine
|
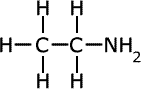
ethylamine
|
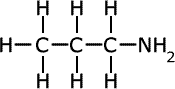
propylamine
|
This prefix form of naming amines is prefectly acceptible as an alternative to the -amine form and indeed comes in very useful when there is more than one amine group to locate on a molecule.
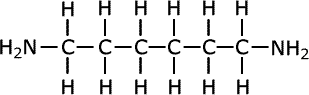
For example, in the manufacture of nylon the compound hexa-1,6-diamine is used: This is also known commonly as 1,6-diaminohexane.
|
hexa-1,6-diamine
|
 |
Secondary amines
Secondary amines may have either two alkyl groups the same on the nitrogen, or two different alkyl chains on the nitrogen. If both alkyl chains are the same they use the prefix 'di-' to indicate it. If the two alkyl chains are different, the name comes from the longest chain, and, if there is any ambiguity as to where the shortest chain is attached, the name shows it tagged onto the nitrogen using the prefix 'N'- to indicate that it is attached to the nitrogen. This is a common way to show where groups are located when heteroatoms are involved.
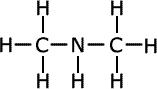
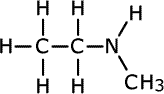
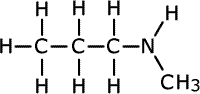
| Structures and names of secondary amines | ||
|---|---|---|
 |
 |
 |
|
dimethylamine
|
methylethylamine
|
N-methylpropylamine
|
In the first structure above, the prefix 'di-' tells us that there are two methyl groups attached to the nitrogen atom in the amine group. In the second structure although there are two different groups attached to the nitrogen, there is no ambiguity as they can't be anywhere else. In the third structure, there are two possible places for the methyl group to be attached once the structure of propylamine has been drawn, so the position of the methyl group must be stated. It is attached directly to the nitrogen of the amine, therefore the name becomes N-methylpropylamine.
Tertiary amines
Tertiary amines have three alkyl groups attached to the nitrogen atom. The same naming convention applies, so that the longest alkyl chain is taken as the root of the name and the other two alkyl groups are added as prefixes. Once again, in case of ambiguity, the position of the alkyl groups is stated using the capital 'N' for nitrogen.
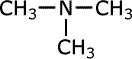
| Structures and names of tertiary amines | ||
|---|---|---|
 |
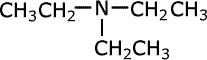 |
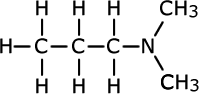 |
|
trimethylamine
|
triethylamine
|
N,N-dimethylpropylamine
|
In the third structure the position of both of the methyl groups must be stated, so the name takes the prefix 'N,N-' to indicate that they are both attached to the nitrogen atom.
Amino acids
Amines are often found in molecules that also have carboxylic acid groups. Such molecules are called amino acids. They form an important group of compounds in living systems, for instance in human biochemistry there are 20 common amino acids that combine to make almost all of the proteins that in a human being.
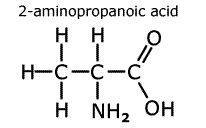 In
naming amino acids, the acid group takes priority and the amine group becomes
a prefix 'amino-'. In the molecule to the left, 2-aminopropanoic acid, the
amine group is located on the second carbon from the carboxylic acid carbon.
This amino acid is also given the trivial name 'alanine'.
In
naming amino acids, the acid group takes priority and the amine group becomes
a prefix 'amino-'. In the molecule to the left, 2-aminopropanoic acid, the
amine group is located on the second carbon from the carboxylic acid carbon.
This amino acid is also given the trivial name 'alanine'.
Some of the common amino acids have much more complicated structures, but they all contain at least one amine group and one acid group.
Amides
Amides are quite distinct from amines even though they do have an -NH2 group. Amides are considered to be derivatives of carboxylic acids as they can be made from them by reaction with ammonia (or ammonium carbonate). They have a carbonyl group attached to the NH2 group of atoms, a carboxamide group.
The synthesis of an amide from a carboxylic acid
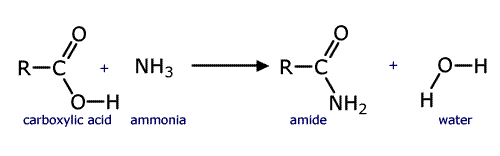
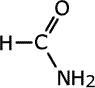
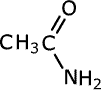
Amides take their names from the longest carbon chain derived from the carboxylic acid. In other words if there are two carbon atoms in the chain, including the carbonyl group, then the root of the name is ethan- (the 'parent' acid is ethanoic acid). The name is completed by tagging on -amide as a suffix.
| Structures and names of amides | ||
|---|---|---|
 |
 |
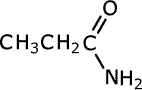 |
|
methanamide
|
ethanamide
|
propanamide
|
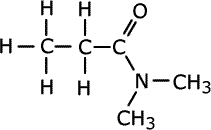
Once again, there may be alkyl groups attached to the nitrogen atom and in case of ambiguity they are prefixed by the letter 'N'.
| An N,N disubstituted amide |
|---|
 |
|
N,N-dimethylpropanamide
|
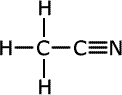
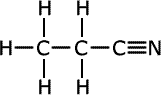
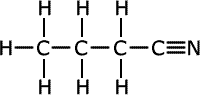
Nitriles
Nitriles contain the -C![]() N
termination at the end of an alkyl chain, called a nitrile group. They are
also considered to be derivatives of carboxylic acids and take their names
from the acid name with the same number of carbon atoms including the carbon
of the nitrile group. Hence, the name of the nitrile with four carbons is
butanonitrile (the 'o' is usually included to make it easier to say)
N
termination at the end of an alkyl chain, called a nitrile group. They are
also considered to be derivatives of carboxylic acids and take their names
from the acid name with the same number of carbon atoms including the carbon
of the nitrile group. Hence, the name of the nitrile with four carbons is
butanonitrile (the 'o' is usually included to make it easier to say)
| Structures and names of nitriles | ||
|---|---|---|
 |
 |
 |
|
ethanonitrile
|
propanonitrile
|
butanonitrile
|
Bridging oxygen atoms
A bridging oxygen atom is one that links together two parts of a molecule.
The two common homologous series that contain oxygen bridges are ethers and esters. Ethers have a simple oxygen bridge between two alkyl groups, -O-.
Ethers take their names from the longest alkyl chain attached to the oxygen, in this case named as if it were an alkane, and the other alkyl chain is added as the prefix - alkoxy (eg methoxy, ethoxy etc.)
Esters have an oxygen as a bridge between a carbonyl group and an alkyl group. R-COO-R'. This carbonyl group with the oxygen makes up the ester linkage, -COO-.
Ethers
Ethers have a bridging oxygen atom between two alkyl chains. The root name is taken from the longest carbon chain, which is named as an alkane, with the shorter of the two chains being a prefix terminating in -oxy.
Essentially the ethers are described as a alkoxy, group, RO-, attached to a hydrocarbon chain.
| Condensed structures and names of the ethers | ||
|---|---|---|
|
|
|
|
|
methoxymethane
|
ethoxyethane
|
methoxyethane
|
Ethers are used as volatile organic solvents.
Ethoxyethane has the trival name 'ether' or 'diethyl ether', both of which are in common usage.
Esters
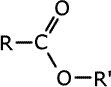 Esters
are carboxylic acid derivatives made by reaction between a carboxylic acid
and an alcohol. They contain the linkage -COO-, in which the carbon atom is
double bonded to one oxygen and single bonded to the other:
Esters
are carboxylic acid derivatives made by reaction between a carboxylic acid
and an alcohol. They contain the linkage -COO-, in which the carbon atom is
double bonded to one oxygen and single bonded to the other:
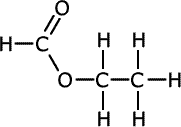
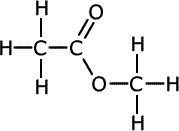
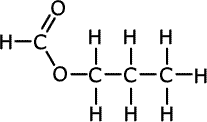
The esters take the root of their name from the carboxylic acid parent and have the alkyl group from the alcohol added at the start as a prefix, - alkyl alkanoate.
| Structures and names of esters | ||
|---|---|---|
 |
 |
 |
|
ethyl methanoate
|
methyl methanoate
|
propyl methanoate
|
In the first structure above, the 'parent' carboxylic acid was methanoic acid, so the structure has the root 'methanoate'. The parent alcohol was ethanol, so the name of the ester becomes ethyl methanoate.