|
This section describes how the standard hydrogen electrode is used to measure the electrode potentials of other redox systems. Syllabus referenceReactivity 3.2.12 - The hydrogen half-cell H+(aq) + e- ⇌ 1/2H2(g) is assigned a standard electrode potential of zero by convention. It is used in the measurement of standard electrode potential, E⦵. (HL)
Guidance
Tools and links |
The standard electrode potential
This is the 'potential' or tendency of a redox system to lose, or gain, electrons when compared to the standard hydrogen electrode - assigned a value of 0 volts. In any reduction-oxidation half-equation the electrons are gained by the species on the left hand side:
Cu2+ + 2e ![]() Cu
Cu
This is an equilibrium, and so if a more powerful reducing agent enters into electrical contact with the above system it can force the copper ions to accept electrons and push the equilibrium to the right hand side. Conversely, if a weaker reducing agent is brought into contact with the above equilibrium then the copper can force it to accept electrons allowing its own equilibrium to move to the left hand side.
The electrode potential measures the tendency of electrons to flow away from or towards a redox equilibrium. They are always measured with respect to the standard hydrogen electrode (which is assigned a value of zero volts).
Equilibrium redox systems with the reduced side (usually a metal) more reactive than hydrogen have a negative electrode potential, i.e. they can lose electrons more easily than hydrogen. Equilibrium redox systems with the reduced side less reactive than hydrogen have a positive electrode potential, i.e. they can lose electrons less easily than hydrogen.
|
Example: The zinc - hydrogen cell Zinc has a standard electrode potential of - 0.76 volts Consequently the equilibrium...
has more of a tendency to move to the right hand side than the equilibrium...
Hence if the two equilibria are brought into electrical contact using an external wire and a salt bridge, the electrons will be pushed from the zinc equilibrium to the hydrogen equilbrium with a force of - 0.76V (the negative sign simply indicates the direction of flow - from zinc to hydrogen ions) The two equations then may be summed together to give the reaction occuring in the whole cell.
overall cell reaction Zn + 2H+ → Zn2+ + H2 |
Using the Standard Hydrogen Electrode
In the actual experimental setup, the two half-cells are connected together under standard conditions, via an external circuit and a salt bridge to make the whole cell.
|
|
In the above apparatus set-up, the zinc|zinc sulfate(aq) half-cell is connected to the SHE via an external circuit which includes a high resistance voltmeter (high resistance to prevent passage of current). The salt bridge completes the circuit - it allows ions to flow from one side to another to equalise the movement of charge.
Once the apparatus is setup, the reading on the high resistance voltmeter records the standard electrode potential of the Zn|Zn2+(aq) system
In this particular case the voltmeter reads -0.76 V, indicating that zinc is more reactive than hydrogen; that there is a force pushing electrons around the external circuit to the hydrogen half-cell from the zinc half-cell.
Cell representation
The whole cell can be represented by showing the half cells in order of phase (solid, | solution, |salt bridge | solution | solid)
The above cell diagram can be represented as:
Zn(s) |Zn2+(aq) ||H+(aq) | H2(g),Pt
By convention the half cell that provides the electrons, i.e. the best reducing agent, is written on the left hand side. In this representation the Zn|Zn2+(aq) is the most negative potential and is behaving as the anode, the electrode where oxidation takes place:
Zn → Zn2+ + 2e
The right hand half cell, H+(aq) | H2(g),Pt, is the electrode where reduction is forced to take place, i.e. the cathode, where electrons are available.
2H+ + 2e → H2
Electrochemical series
By comparing many redox systems with the SHE and other reference electrodes, a series can be drawn up showing the reductions in order of their standard electrode potential (usually from negative at the top to positive at the bottom)
Using the electrochemical series, the species at the top on the right hand side are reducing agents, and the species at the bottom on the left hand side are oxidising agents.
|
redox half-equation
|
Eº
/ V
|
| Mg2+(aq) + 2e- → Mg(s) |
|
| Zn2+(aq) + 2e- → Zn(s) |
|
| Fe2+(aq) + 2e- → Fe(s) |
|
| Pb2+(aq) + 2e- → Pb(s) |
|
| 2H+(aq) + 2e- → H2(g) |
|
| Sn4+(aq) + 2e- → Sn2+(aq) |
|
| Cu2+(aq) + 2e- → Cu(s) |
|
| Ag+(aq) + e- → Ag(s) |
|
Cl2(g) + 2e-  2Cl-(aq) 2Cl-(aq) |
|
The value of the electrode potential is a relative value for the equilibrium compared with the standard hydrogen electrode. Its sign never changes regardless of which way round the equilibrium is written. By convention, the equilibria are written as reductions (left to right) but they could just as easily be written as oxidations, without changing the sign of the electrode potential.
Full standard electrode potentials table
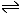 Zn2+ + 2e
Zn2+ + 2e 
