|
Some molecules cannot be easily described using the octet rule, as they have more than four electron pairs around the central atom. This section explores the phenomenon of octet expansion. |
 |
Octet expansion
The elements in period 3 of the periodic table can use available 'd' orbitals to increase the number of electrons that can be accomodated in the outer valence shell. This only occurs when covalent bonding takes place involving relatively electronegative elements, such as oxygen, fluorine and chlorine.
This increase in the valence shell electrons is known as 'octet expansion' and it gives rise to molecules that have more than four charge centres on the central atom.
Octet expansion can also occur in ions.
|
Molecules with octet expansion
|
Ions with octet expansion
|
|
PCl5, SF6, XeF4,
XeF6
|
PCl6-, SF5+,
|
Although the actual theory is not needed at IB level, it is useful to understand how orbital systems can exist with more than four charge centres. The central atom 'borrows' orbitals from the 3d level (see) and hybridises them along with the 3s and 3p orbitals, to produce the number of orbitals required to share electrons with covalently bonded atoms. Thus, sp3d2 (six orbitals) can be used to make an octahedral system.
Electronic shapes of five and six electron domain centres
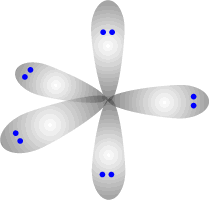
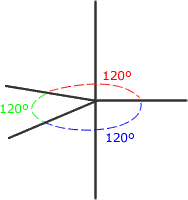
There is no perfect shape that allows five regions of electron density to be equally separated from one another. The shape adopted is trigonal bipyramidal. This involves a trigonal plane with two axial regions at 90º (perpendicular) to the plane.
|
Trigonal bipyramidal shape
|
The electron regions repel as
far as possible
|
 |
 |
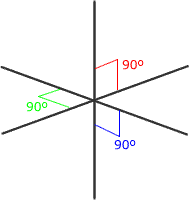
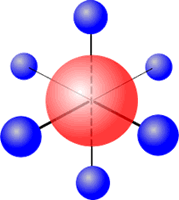
Six regions of electronic charge can be accomodated into a perfect octahedral shape.
The name 'octahedral' seems strange at first sight, as the 'octa' suggests that there are eight regions. The name 'octahedral' actually means 'eight faces, or sides'. Each side is produced by joining the ends of three axes.
|
Octahedral shape
|
The electron regions repel as
far as possible
|
 |
 |
Five electron domain systems
Five charge centres must involve a minimum of 10 electrons. Group 15 elements have 5 outer (valence) electrons. When bonded to five atoms this makes a total of 10 electrons = five shared pair charge centres.
|
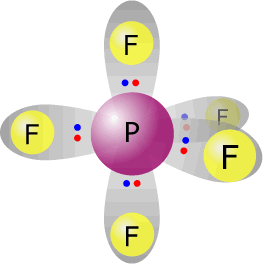
phosphorus(V) fluoride - PF5 |
|
|---|---|
|
Phosphorus (group 15) has 5 valence electrons Each fluorine provides one electron, there are 5 fluorines therefore 5 electrons. Total electrons = 10 = 5 pairs All pairs used for bonding therefore molecular shape = electronic orientation. Trigonal bipyramidal |
|
For molecules, or ions, with five electron domains, but only four attached atoms, there are two possible positions for the lone pair of electrons. It could either be in the axial (top or bottom) position of the trigonal pyramid, or it could be on one of the equatorial positions. The disadvantage of an axial position is that this leaves the lone pair at 90º to three bonding pairs, whereas if an equatorial position is adopted, it leaves the lone pair at 90º to only two other bonding pairs. This second alternative is the lowest energy in terms of repulsion and therefore the one adopted.
|
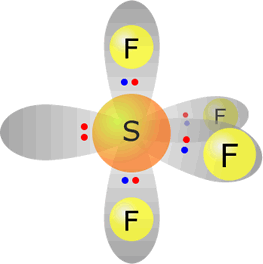
sulfur(IV) fluoride - SF4 |
|
|---|---|
|
Sulfur (group 16) has 6 valence electrons Each fluorine provides one electron, there are 4 fluorines therefore 4 electrons. Total electrons = 10 = 5 pairs, therefore electronically the pairs adopt a trigonal bipyramidal orientation. However, only four pairs are used for bonding, therefore molecular shape: sawhorse |
 |
Six charge centre systems
Six charge centres adopt an octahedral orientation and when all six electron pairs are used for bonding it gives rise to an octahedral molecule, for example, SF6.
|
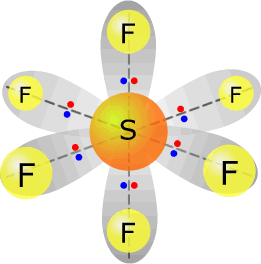
sulfur(VI) fluoride - SF6 |
|
|---|---|
|
Sulfur (group 16) has 6 valence electrons Each fluorine provides one electron, there are 6 fluorines, therefore 6 electrons. Total electrons = 12 = 6 pairs, therefore electronically the pairs adopt a trigonal bipyramidal orientation. All six pairs are used for bonding, therefore molecular shape: octahedral |
 |
When one of the electrons pairs is not used for bonding (lone or non-bonding pair) the remaining shape is a five coordinated, square pyramidal structure. One of the attached atoms is the top of the pyramid and the other four make up the base. An example is iodine(V) fluoride.
|
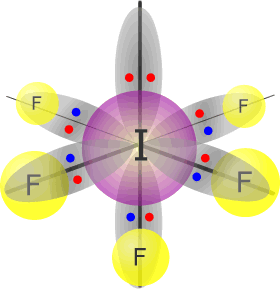
Iodine(V) fluoride - IF5 |
|
|---|---|
|
Iodine (group 17) has 7 valence electrons Each fluorine atom provides 1 electron, there are 5 fluorines, therefore 5 more electrons. Total electrons = 12 = 6 pairs, therefore electronically, the pairs adopt a octahedral arrangement. One of the electron pairs is not used for bonding. umbrella shape |
 |
When five bonding pairs are involved, there is no specific advantage, or disadvantage, for the lone pair as regards the position it adopts as the octahedral shape is totally symmetrical, however when only four pairs of electrons are used for bonding the two remaining lone pairs arrange themselves at 180º to one another.
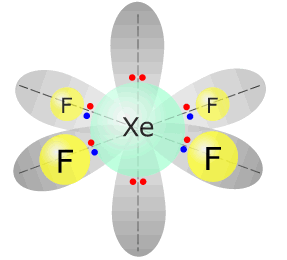
This leaves the remaining bonding pairs in a square planar shape, for example XeF4.
|
Xenon tetrafluoride - XeF4 |
|
|---|---|
|
Xenon (group 18) has 8 valence electrons Each fluorine atom provides 1 electron, there are 4 fluorines, therefore 4 more electrons. Total electrons = 12 = 6 pairs, therefore electronically, the pairs adopt a octahedral arrangement. Two of the electron pairs are not used for bonding. square planar |
 |
Summary - determination of molecular shape
1. Count up the total valence electrons on the central atom. This is done by consideration of the group number of the central atom and the number of attached atoms.
2. The total number of electron pairs gives the electronic arrangement adopted:
- two: linear
- three: trigonal planar
- four tetrahedral
- five: trigonal bipyramidal
- six: octahedral
3. Each of the atoms bonds to the central atom by means of a bonding pair of electrons. If there are no electron pairs remaining, then the molecular shape is the same as the electronic arrangement. If there are any lone pairs, then only the bonding pairs are considered in the molecular shape. The lone pairs may distort the final shape in some cases, due to greater repulsions between lone pairs and bonding pairs than between bonding pairs and bonding pairs.
| Total electron pairs | number of lone pairs | electronic arrangement | molecular shape |
|---|---|---|---|
| 2 | 0 | linear | linear |
| 3 | 0 | trigonal planar | trigonal planar |
| 3 | 1 | trigonal planar | angular |
| 4 | 0 | tetrahedral | tetrahedral |
| 4 | 1 | tetrahedral | pyramidal |
| 4 | 2 | tetrahedral | angular |
| 5 | 0 | trigonal bipyramidal | trigonal bipyramidal |
| 5 | 1 | trigonal bipyramidal | saw-horse |
| 5 | 2 | trigonal bipyramidal | distorted T-shaped |
| 5 | 3 | trigonal bipyramidal | linear |
| 6 | 0 | octahedral | octahedral |
| 6 | 1 | octahedral | umbrella |
| 6 | 2 | octahedral | square planar |

