|
There are many different types of indicators. Although they may all be used for pH detection they are not all suitable for the same purpose. |
|
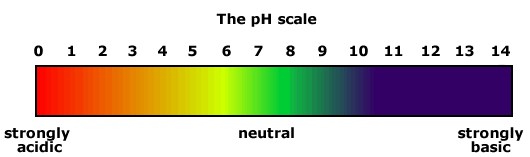
Universal indicator
Universal indicator is a mixture of coloured compounds, which is used for simple testing of solutions. It is of no use for titrations as there are several colour changes that take place over a variety of pH values.
Universal indicator is also available in the form of test paper. This consists of strips of paper impregnated with universal indicator solution and dried. It may be used to test solutions or gases. To test gases the paper must be dampened previously wth a drop of water and then held in the gas.
Litmus
Litmus is a vegetable based dye that was used in schools before the advent of universal indicator. It has a simple colour scheme, in acids it turns red and in bases it turns blue, however the mid-point is not sharply defined and is unsuitable for titrations.
It is usually used in the form of paper impregnated with the litmus dye. This comes in two varieties, the red paper that is used to test for bases and the blue litmus paper that is used to test for acids.
Litmus paper when previously dampened can also be used to test for the acidity of gases.
Phenolphthalein
Phenolphthalein is an organic indicator with a pKa value of 9.3. It is red in bases and colourless in acids.
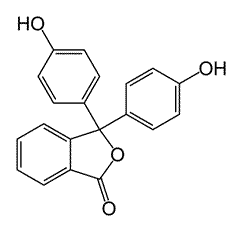 |
Although it has a complicated structure, the good news is that you don't need to know it! In basic solution the molecule loses a hydrogen ion and becomes a negative ion. This can be represented by the much simpler equation:
The molecular form of phenolphthalein is represented as HIn and the ionic form as In-. When base is added it removes the hydrogen ions from the equilibrium and the equation moves to the right hand side. We see the colour of the anion, i.e. red. |
One slight complication of phenolphthalein is that the colour tends to fade as new products are formed on standing. In titrations the readings at the end point should be taken fairly quickly if adding base to acid.
Phenolphalein is the indicator of choice when titrating weak acids with strong bases, for example, ethanoic acid with sodium hydroxide.
Methyl orange
Methyl orange is an acid base indicator that turns red in acidic solution and yellow in base, the mid-point colour is orange. Its pKa value is 3.4.
The fact that its pKa lies in the acidic region of the pH scale makes methyl orange useful for titrations involving weak bases and strong acids, such as ammonia solution and hydrochloric acid. The equivalence point of this titration also lies in the acidic region and so the colour change is marked when the mixture passes through the neutralisation point.
 |
 |
 |
|
Methyl orange in acidic solution
|
Methyl orange at the equivalence point
|
Methyl orange in base solution |
Bromothymol blue
Bromothymol blue is an acid base indicator which turns yellow in acid solution and blue in base. It has a pKa of 7.0 and consequently is used for titrations involving strong acids and strong bases.
Choice of indicator summary
As discussed above, the indicator must be chosen to suit the titration being performed.
Titrations involving strong acids and bases can be performed using any indicator as the pH change at the equivalence point is large (from 3 to 11 or vice versa) - most indicators operate within this range.
Titrations involving strong acids and weak bases have an equivalence point in the acidic region of the pH scale. The choice of indicator will depend on the actual expected pH at the equivalence point. It makes sense to select an indicator with a pKa right in the middle of the pH change at the equivalence point. In practice, there are few indicators in common use. For strong acids and weak bases methyl orange is the indicator of choice.
| Indicator |
pKa
|
Useful range
|
| Methyl orange |
3.7
|
3.1 - 4.4
|
| Bromophenol blue |
4.0
|
3.0 - 4.6
|
| Methyl red |
5.1
|
4.2 - 6.3
|
| Bromothymol blue |
7.0
|
6.0 - 7.6
|
| Phenol red |
7.9
|
6.8 - 8.4
|
| Phenolphthlein |
9.3
|
8.2 - 10.0
|