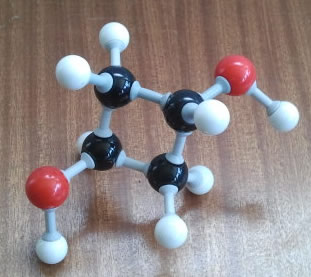
All of the following questions refer to the picture of the molecular model on the right hand side:
(i) Draw the full structural formula
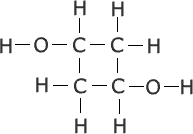 |
(ii) Write out the molecular formula.
| C4H8O2 |
(iii) Write out the condensed structural formula representation.
| C4H6(OH)2 - (rings are kept intact in the representation. In this case the C4H6 represents the hydrocarbon ring) |
(iv) What is the full systematic name of the molecule?
| trans-cyclobutan-1,3-diol |
(v) What kind(s) of intermolecular forces does this molecule experience?
| Dispersion forces (induced dipole-dipole), Hydrogen bonding. Can you see why there is no permanent dipole? |