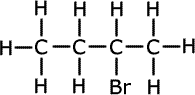
Two reactions of an alkene, B, are shown below.
[A] C4H10 <---(H2/Ni)------ [B] CH2-CH=CH-CH2 (trans) ------> [C] C4H9Br
a) State the name of A and write an equation for its complete combustion. Explain why the incomplete combustion of A is dangerous.
ans a) (i)|
Compound A is butane. Combustion: 2C4H10 + 13O2 Incomplete combustion produces carbon monoxide gas, which is colourless odourless and poisonous. |
ans a) (ii)ii) Outline a test to distinguish between A and B stating the result in each case.
|
B is an alkene and will turn bromine water from orange to colourless. A is an alkane and as such, has no effect on bromine water. |
ans a) (iii)iii) Write an equation for the conversion of B to C. State the type of reaction taking place and draw the structure of C.
|
CH2-CH=CH-CH2 + HBr This is an example of electrophilic addition.
|
b) A compound D has a molecular formula C2H4O2 and is obtained from a reaction between methanol and methanoic acid. Write an equation for this reaction and state the name of D.
ans (b)|
CH3OH + HCOOH D is methyl methanoate |
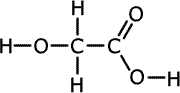
c) A second compound E has the same molecular formula as D and has acidic properties. State the name of compound E.
ans (c)|
The acidic isomer of methyl methanoate is ethanoic acid, CH3COOH. |
d) The first synthetic thread was made from a polyester. A section of the polyester is drawn below:
-----CH2COO------CH2COO-----CH2COO-----
Give the structural formula of the monomer (containing two functional groups) that could be used to make this polyester and state the names of the two functional groups.
ans (d)|
The polymer contains ester linkages, and so must be formed from condensation (esterification) of a carboxylic acid group and an alcohol group.
|
e) State, giving a reason, whether this polyester is made by a condensation or an addition reaction.
ans (e)|
The polymerisation reaction consists of addition of two molecules, followed by elimination of a water molecule each time the polymer adds another monomer unit. Addition elimination is also called condensation. |