The compound C2H4 can be used as a starting material for the preparation of many substances.
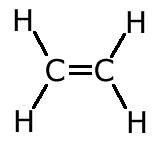
ans (a)a) name the compound C2H4 and draw its structural formula.
|
C2H4 is ethene.
|
ans (b)b) In the reaction scheme below state the type of reaction and identify the reagent needed for each reaction.
C2H4
CH3CH2OH
CH3COOH
|
Step 1: C2H4 Step 2: CH3CH2OH |
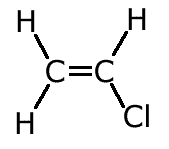
ans (c)c) C2H4 can be converted into one of the following compounds in a single step reaction C2H3Cl or C2H4Cl1.
Draw the structural formula for each of these compounds and identify the compound that can be formed directly from C2H4.
|
Structural formulae:
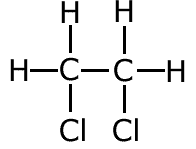
Compound II, dichloroethane can be formed directly from ethene by reaction with chlorine. CH2=CH2 + Cl2 |
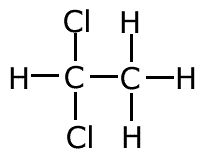
d) one of the compounds in c) has an isomer. Draw the structural formula of the isomer and explain why it cannot be formed directly from C2H4.
ans (d)|
The compound 1,2-dichloroethane has an isomer, 1,1-dichloroethane which cannot be made from ethene.
The reason is that the chlorine molecule must add to the ethene across the double bond, with one chlorine atom going to each of the two carbons. |
e) C2H4 can also react to form a polymer. Name this type of polymer and draw the structural formula of a section of this polymer consisting of three repeating units.
ans (e)|
Ethene undergoes addition polymerisation. The product is called an addition polymer. CH2=CH2 Three repeating units: -(-CH2-CH2-)-(-CH2-CH2-)-(-CH2-CH2-)- |
f) Polymers can also be formed in a different type of reaction. Identify this type of reaction and name two different types of such polymers.
ans (f)|
The other form of polymerisation is condensation polymerisation. Two different kinds of condensation polymers: polyester and polyamides |
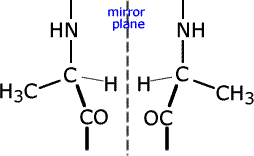
g) The polymer with the repeating unit -(-NH-CH(CH3)-CO-)- exists as optical isomers.
ans (g)(i) State a test for optical isomers
(ii) Identify the chiral centre in the repeating unit
(iii) Draw the two enantiomeric forms of the repeating unit.
|
(i) Optical isomers rotate the plane of polarised light. (ii) the chiral (asymmetric) centre (carbon atom) has four different groups attached, -(-NH-CH(CH3)-CO-)- (iii) |