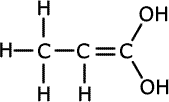
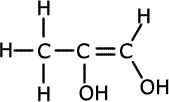
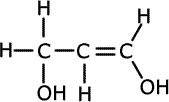
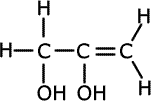
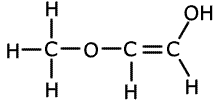
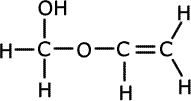
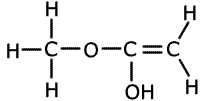
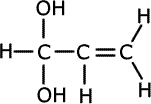1. Several compounds have the molecular formula C3H6O2, three of them have the following properties:
A is soluble in water and is acidic. B and C are neutral and do not react with bromine.
a) Give the structural formula for each of these compounds and name them.ans (a)
|
Solubility in water suggests hydrogen bonding, alcohol or carboxylic acid. The acidic nature of A tells us that it is a carboxyic acid. B and C do not react with bromine, so they have no double bonds between carbon atoms. The double bonds must therefore be between carbon and oxygen atoms. Esters are isomers of carboxlic acids so these are the molecules of choice.
There are other structures possible for B and C, such as 2-hydroxypropanal, 3-hydroxypropanal and hydroxypropanone. |
ans b)(i)b) (i) explain the solubility and acidity of A in water.
|
The carboxylic acid group dissociates in water making carboxylate ions. These are easily hydrated by the water molecules, making carboxylic acids soluble. The same dissociation releases hydrogen ions into the water. These are responsible for acidity. C2H5COOH |
b) (ii) Write and equation for the reaction of A with sodium hydroxide solutionans b) (ii)
|
C2H5COOH + NaOH |
b) (iii) explain why B and C do not react with bromine.
ans b)(iii)| Neither B nor C contain a double bond. Bromine adds to double bonds via electrophilic addition. |
c) State and explain which one of A, B and C has the highest boiling point.
ans (c)|
Melting points are a good measure of the degree of intermolecular bonding that occurs in a compound. Carboxylic acids have strong hydrogen bonds between molecules, increasing their melting and boiling points. Therefore A, propanoic acid, has the highest boiling and melting point. |
d) (i) Name the class of compounds to which B and C belong and state a use for this class of compounds.
ans d)(i)|
B and C belong to the homologous series of esters. They are used in the food and perfume industries as flavourings and scents. |
d) (ii) Name the two classes of compounds used to form B and C and state the other product formed in the reaction.
ans d)(ii)|
Esters can be made from the reaction between carboxlic acids and alcohols. The other product is water. Alcohol + carboxylic acid |
e) (i) Suggest the structural formula of an isomer of C3H6O2 which does react rapidly with bromine. Name this type of reaction and describe an observation that can be made during the reaction.
ans (e)|
Any suitable structure with a double bond between carbon atoms, such as the propene-diols, or methoxyethene-alcohols
methoxyethene-alcohols (complicated nomenclature)
The type of reaction ndergone between double bonds and bromine is called electrophilic addition. The bromine loses colour as it adds to the double bond, so the colour change is red/orange to colourless. |
.gif)
.gif)
.gif)