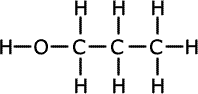
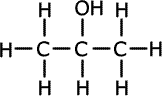
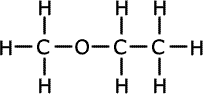
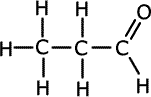
1.a) This question is concerned with compounds of formula C3H8O.
ans (i)i) Draw the full structural formula of the three possible isomers and give the name of each.
|
ans (ii)ii) Predict how each of these isomers would behave when reacted with limited (i.e. not in excess) acidified potassium dichromate(VI) solution and describe any observations that could be made. Write the structures of any organic product formed and give their names.
|
Propan-1-ol forms propanal Propan-2-ol forms propanone Methoxyethane has no reaction n the course of the oxidation reactions the potassium dichromate(VI) turns from orange to green.
|
ans (iii)iii) The infrared spectrum of one of the three possible isomers shows an absorption band at 1000-1300cm-1, but no absorption band above 3000cm-1. Use the data table to state to which of these isomers this spectrum can be assigned and give your reasoning.
Two of these isomers can be dehydrated to give the same product. Identify the two isomers and give the structure of the product. Give an equation for a characteristic reaction of the product.
|
The isomer has a C-O bond as shown by the absorption at 1000-1300cm-1, but is not an alcohol as there is no absorption above 3000cm-1. Therefore the spectrum belongs the the methoxyethane. The two isomers that give the same dehydration product, an alkene, are propan-1-ol and propan-2-ol.
A characteristic reaction of alkenes is the decolorisation of bromine water, an addition reaction. CH3CHCH2 + Br2 |
b) Two of these isomers give the following NMR spectra.
- Spectrum A with peak areas in the ration 3:1.2:1
- Spectrum B with peak areas in the ratio 6:1:1
Assign each of these spectra to one of these isomers you have drawn and outline your reasoning.
ans (b)
Spectrum A corresponds to propan-1-ol as there are four different environments for the hydrogen atoms. Spectrum B corresponds to propan-2-ol as the two CH3 groups are equivalent, so there are only three different hydrogen environments. |