1.a) Some organic compounds can undergo dehydration
ans (i)i) State what is meant by the term dehydration and give an example of a dehydrating agent.
|
Dehydration means loss of the atoms of water, i.e. loss of two hydrogen atoms and one oxygen atom, usually from adjacent carbon atoms. sulfuric acid and phosphoric acid are good dehydrating agents. |
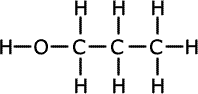
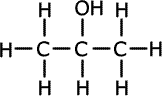
ans (ii)ii) Two of the isomers of molecular formula C3H8O can be dehydrated to form a compound of molecular formula C3H6. Give the structural formula and the names of these two compounds.
|
Alcohols are dehydrated to give alkenes. Therefore to produce propene we need propanol. The following are possible isomers that could be dehydrated to propene:
|
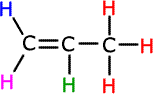
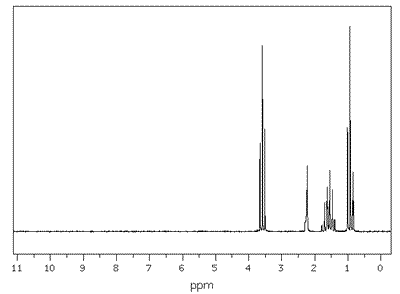
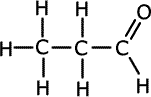
ans (iii)iii) State the number of peaks and the ratios of their areas in the 1H NMR spectra of C3H6 and one of the isomers of C3H8O
Use the data table to identify a strong absorption in the infrared spectrum of C3H8O, which is not present in C3H6 and a strong absorption in C3H6 which is not present in C3H8O. In each case state the absorption range and the bond responsible.
|
Propene has four different hydrogen environments (each shown below with a different colour) and therefore four separate peaks:
Propan-1-ol also has four different environments and four separate peaks in the nmr spectrum. From The IR data there is an absorption expected in propene, due to the double bond, at 1610 to 1680cm-1, and in propan-1-ol there is an absorption at 3230 to 3350cm-1, due to the presence of the hydrogen bonded -OH group. |
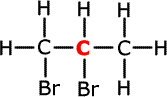
b) The compound C3H6 can react with bromine. Write an
equation for this reaction and name the product. State a visible change which
accompanies the reaction.
|
C3H6 + Br2 The product is 1,2-dibromopropane. The bromine (orange/red) forms a colourless product. |
c) Give the full structural formula of the product formed in part
(d) (ii) and identify the chiral carbon atom.
|
d) State what distinctive property a chiral carbon atom gives to
a molecule.
|
Chiral carbon atoms produce molecules with optical isomers. 1,2-dibromopropane has two enantiomers. |
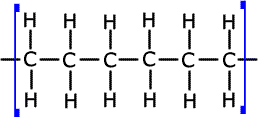
e) Name the type of polymerisation reaction that C3H6
undergoes and draw the structure of a section of the polymer chain formed from
three monomer molecules.
|
Propene is an alkene and forms addition polymers. Below is the section of the addition polymer showing three monomer molecules.
|
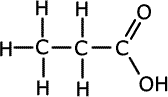
f) One of the isomers of formula C3H8O can
be oxidised to form two different organic products, depending on the conditions
used.
Identify an appropriate oxidising agent, give the structures of the two products
and specify the conditions required for the formation of each.
|
Primary alcohlos can form two different products on oxidation, depending on the conditions. The oxidising agent of choice is potassium dichromate (VI) in acidic conditions (usually dilute sulfuric acid).
|