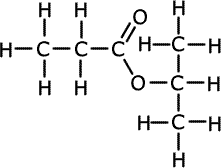
1.a) A pleasant smelling liquid A was hydrolysed in acid conditions to give two organic products B and C. Product B, a carboxylic acid was readily soluble in water. The mass spectrum of B is shown below.
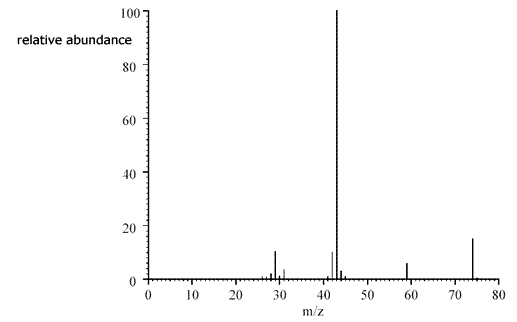
ans (i)i) Identify the Mr of B. Suggest fragment ions responsible for the peaks at m/e 29, 45, and 57
|
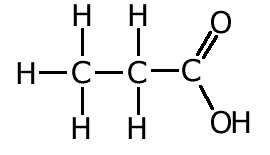
Using the peak due to the molecular ion (the peak at the highest m/e ratio), the relative molecular mass = 74. B is a carboxylic acid, therefore contains the COOH group (probable formula C2H5COOH). This group can form an ion [COOH]+ with an m/e ratio = 45. If 45 is subtracted from the relative mass of 74 we get a fragment with mass = 74 - 45 = 29. This is due to [C2H5]+ The fragment at 57 suggests a loss of a group with a masss of 74 - 57 = 17. This corresponds to loss of [OH] leaving a fragment [C2H5CO]+. |
ans (ii)ii) Using the information in (i) deduce the structural formula of B.
|
|
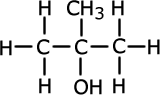
ans (iii)iii) Product C was also soluble in water and on analysis was found to contain: 60% carbon, 13.3 % hydrogen and 26.7% oxygen by mass.
The Mr of C is 60. Calculate the empirical and molecular formula of C.
|
Divide the percentages by the relative mass of each atom: C = 5 : H = 13.3 : O = 1.67 Divide through by the smallest number: C = 3 : H = 8 : O = 1 Empirical formula = C3H8O This empirical fromula has a mass = 36 + 8 + 16 = 60. This is the same as the given molecular mass of C, therefore the empirical formula is also the molecular formula = C3H8O |
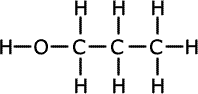
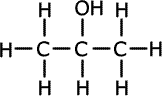
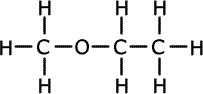
b) Draw three possible structural formula for the isomers with
this molecular formula.
Explain why two of these isomers shows a broad absorption in the IR spectrum,
which will be missing in the spectrum of the third isomer.
Both of the alcohol isomers show a strong absorption in the IR spectrum due to the -OH group. The ethoxyethane (an ether) does not contain an -OH group. |
c) Explain how the two isomers with the broad absorptions in the IR spectrum could be identified from their 1H NMR spectra.
ans (c)|
The NMR spectrum identifies equivalent hydrogen atoms. In the propan-1-ol there are four sets of hydrogen atoms in different environments (CH3, CH2, CH2, OH) whereas in the propan-2-ol there are only three distinct environments ((CH3)2, CH, OH) |
d) Compound C was oxidized to compound D by refluxing with acidified
sodium dichromate(VI) solution. D was not acidic but contained the same number
of carbon atoms as C. Deduce the structural formula of C and D and give names
for each.
|
Alcohols are oxidised by sodium dichromate(VI) in acid to either aldehydes or ketones, depending on the nature of the alcohol. Primary alcohols first oxidise to aldehydes and then further to carboxylic acids. Secondary alcohols are oxidised to ketones and no further oxidation can take place. In this case there is no acidity detected so the product is not a carboxylic acid therefore it must be a ketone. It follows then that the alcohol was secondary. Therefore:
|
e) Deduce the structural formula of A
|
A pleasant smelling liquid A was hydrolysed in acid conditions to give two organic products B and C B has been identified as propanoic acid and C has been identified as propan-2-ol. These are made by hydrolysis of A, therefore A is the ester of propanoic acid and propan-2-ol.
|
f) An alcohol E does not react with acidified potassium dichromate (VI) but forms an alkene, F, with a molecular formula C4H8, when heated with concentrated sulfuric acid. Deduce the structures and state the names of E and F.
ans (f)|
If the alcohol does NOT react with acidified potassium dichromate(VI) then it must be a tertiary alcohol. The same alcohol dehydrates when heated with conc. H2SO4 to give a structure with molecular fromula C4H8, therefore it comes from the tertiary alcohol with four carbon atoms. There is only one, methylpropan-2-ol.
|
g) Write and equation for the conversion of E to F and state the type of reaction that occurs.
ans (g)|
This is a dehydration reaction: CH3C(OH)(CH3)2 |