1.a) Halogenoalkanes undergo nucleophilic substitution reactions. The rates
and mechanisms of these reactions depend on whether the halogenoalkane is primary
secondary or tertiary.
ans (i)i) Explain the term nucleophilic substitution.
|
Nucleophilic substitution is when the organic molecule is attacked at the positive atom of a dipole by a reagent that has a lone pair of electrons, resulting in replacement (substitution) of an atom or group by another. |
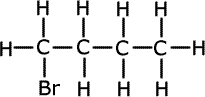
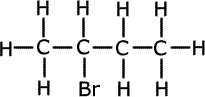
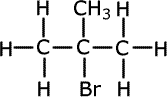
ii) The formula C4H9Br represents more than one compound. Using this formula draw a structure (showing all bonds) to represent a halogenoalkane that is:
-
Primary
-
Secondary
-
Tertiary
|
ans (iii)iii) The stoichiometric equation for a nucleophilic substitution reaction is given below:
(CH3)3CBr + OH-(CH3)3COH + Br-
The reaction takes place via a two step mechanism. Write an equation for each step.
|
Step 1: (CH3)3CBr Step 2: (CH3)3C |
b) Define the following terms
ans (b)(i) Molecularity
| The molecularity is the number of particles involved in the rate determining step of the reaction mechanism. |
ans (c)(ii) Rate determining step
|
The rate determiniing step is the slowest step of the mechanism. |
d) Identify the rate determining step in the mechanism above
ans (d)|
The rate determining step is the first step. |