|
Electrochemical cells convert chemical energy into electrical energy using redox reactions. There are many potential experiments that could be carried out using the variables involved in the electrochemical apparatus set up. |
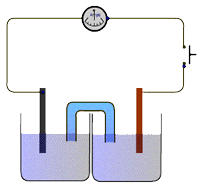 |
The apparatus
The electrochemical cell consists of two half-cells connected to a high resistance voltmeter in the external circuit and the solutions connected via a salt bridge.
The salt bridge consists of a tube (or filter paper) containing saturated potassium nitrate solution or gel. The potential difference between the two half-cells is recorded on the high resistance voltmeter.
The standard cells have been extensively studied and theoretical values for the potential difference between the two half-cells are well known.
For example, the Daniel cell in the diagram above should provide a potential difference of 1.10V under standard conditions of 1 mol dm-3 solutions and a temperature of 298K
Possible variables to investigate are:
- The temperature
- The half-cell concentration
- The salt bridge length
- The concentration of potassium nitrate in the salt bridge
- The effect of changing the half-cells by chosing different metal || metal salt combinations
Whichever variable is chosen to investigate, the important thing is to make sure that it is monitored throughout the experiment. Likewise, the controlled variabled must be monitored to ensure that they do not change. In this experiment it is clear that the output (or dependent) variable is the potential difference recorded on the high resistance voltmeter.
