|
Aldehydes and ketones are both carbonyl compounds, that is they contain alkyl chains attached to a C=O group. The difference between them is that the aldehyde also has a hydrogen attached to the carbonyl group. This confers aldehydes with sightly different properties to ketones. |
Aldehydes
Aldehydes have the general formula CxH2x+1CHO, although the first member of the homologous series is methanal, HCHO (x=0).
The carbonyl group has a pair of electrons in a pi orbital between the carbon atom and the oxygen of the carbonyl group. This is a polarised system due to the high electronegativity of the oxygen atom.

Oxidation
This reactivity of the carbonyl group means that aldehydes can be oxidised easily to carboxylic acids. This is carried out using potassium dichromate(VI) in acidic solution under reflux (to prevent loss of the volatile aldehyde).
Reduction
In section section 10.33 the oxidation of alcohols to aldehydes was described:
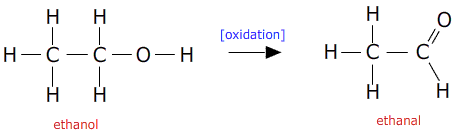
This reaction can be made to go in the reverse direction using a strong reducing agent.
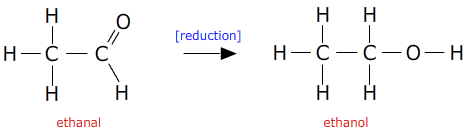
Suitable reducing agents are lithium aluminium hydride (lithium tetrahydroaluminate) or sodium borohydride (sodium tetrahydroborate).
Lithium aluminium hydride (lithium tetrahydroaluminate)
Lithium aluminium hydride, LiAlH4, is a highly reactive reducing agent that must be used in non-aqueous solutions, for example, ethoxyethane (ether). This is because it reacts vigorously with water.
The reaction is carried out in two stages:
- 1 heating with LiAlH4 in ether forming a complex
- 2 decomposition of the complex formed in 1 by adding aqueous acid to give the required product
Reduction using lithium aluminium hydride
Sodium borohydride (sodium tetrahydroborate)
Sodium borohydride, NaBH4, has an advantage over lithium aluminium hydride in that it is not decomposed by water at high pH and hence can be used in aqueous solutions.
It is, however, a less powerful reducing agent, although perfectly adequate for reduction of aldehydes and ketones.
Ketones
Ketones have the general formula CxH(2x+2)CO, arranged with the two alkyl groups either side of the carbonyl group.
Ketones cannot undergo oxidation without cleavage of a carbon - carbon bond, which would require extreme conditions. With potassium dichromate(VI) in acidic solution there is no reaction.
Reduction of ketones
In section 10.33 the oxidation of secondary alcohols was shown to produce ketones. Using a suitable reducing agent the reverse reaction is also possible. Hence, ketones are reduced to secondary alcohols by strong reducing agents:
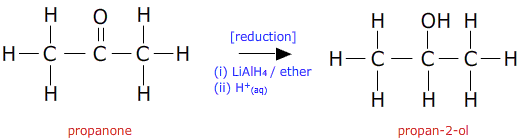
As with aldehydes, both lithium aluminium hydride and sodium borohydride (sodium tetrahydroborate) may be used.

