|
This form of isomerism is caused by an inability to internally rotate molecules effectively "fixing" the positions of atoms in specific positions. When there are different substituents this can give rise to non-superimposible isomers (stereoisomers) |
|
Stereoisomerism
'Stereo' - from two positions in space.
This is when two molecules have the same molecular formula and the same groups of atoms, but they are arranged in such a way that different forms of the molecule are non-superimposible.
There are two types of stereoisomerism:
- Cis-trans or E/Z isomerism
- Optical isomerism.
Stereoisomers have the same molecular formula and the same systematic name.
Cis-trans isomerism
Cis-trans isomerism is caused by the molecule having the same basic structure, but a different geometry (don't let the word put you off, it's not difficult). This occurs when groups or atoms are fixed on one side of a carbon chain by a lack of rotation between two carbon atoms in the chain, usually caused by a double bond.
Isomers are named by using the prefix 'cis' to indicate that important groups are on the same side of the chain, or 'trans' to indicate that the important groups or atoms lie on opposite sides of the carbon chain.
In the diagram above, the 'cis' isomer has two chlorine atoms which lie on the same side of the carbon chain, (the bottom, as we look at it), while the 'trans' isomer has the two chlorine atoms on opposite sides of the carbon chain.
The relationship between the atoms within each structure is unique, giving rise to different chemical and physical properties. This is true for all geometric isomers.
Alkenes
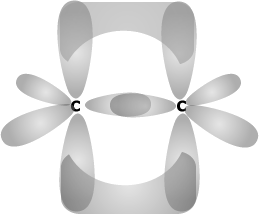
The most important group of compounds that exhibit geometric isomerism is the alkenes. There is no rotation about a double bond due to the nature of the 'pi' bond system, which would break if the molecule were to rotate about the carbon - carbon bond.
 |
 |
|
The 'pi' molecular orbital system arises by lateral
overlap of parallel 'p' orbitals.
|
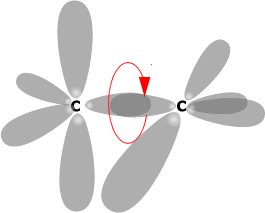
If rotation happens about the C-C (sigma bond) then
the 'p' orbitals can no longer overlap.
|
Cyclic molecules
Other systems with restricted rotation are cyclic molecules. If rotation were to be attempted about a carbon-carbon bond it would break the molecule. This also gives rise to geometric isomerism.
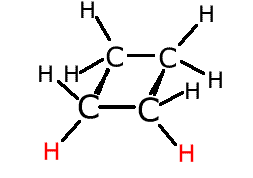 |
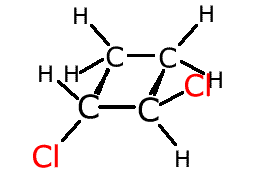
In the cyclobutane molecule, left, the hydrogen atoms at the front (red) are both below the plane of the ring. They are fixed in this position by a lack of rotation between the two carbon atoms at the front. If two different groups replace these two red hydrogen atoms this gives rise to geometric isomerism. |
If the different atoms cross from under the ring plane to over the ring plane the molecule is referred to as 'trans'. If the different attachments both lie on the same side of the ring plane they are referred to as 'cis'.
|
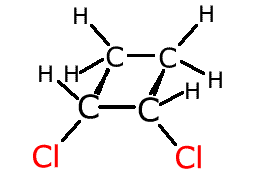
cis-1,2-dichlorocyclobutane
|
trans-1,2-dichlorocyclobutane
|
 |
 |
Physical properties
Geometric isomers differ in density, viscocity, refractive index, enthalpy of vaporisation and they also have different melting and boiling points. The arrangement of the atoms in space generates varying degrees of intermolecular attractions, giving rise to differences in the physical properties.
|
cis-but-2-ene
|
trans-but-2-ene
|
|
|
m.p./K
|
134
|
167
|
|
b.p./K
|
277
|
274
|
|
ΔH(vap)
|
22.7 kJ mol-1
|
22.0 kJ mol-1
|
Chemical properties
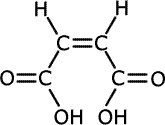
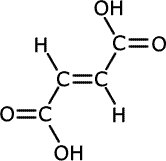
The double bond holds groups in specific orientations, having an effect on the chemical properties of geometric isomers. The classic case is that of the butenedioic acids, HOOCCH=CHCOOH. They have the following structures:
 |
 |
|
cis-butenedioic acid
|
trans-butenedioic acid
|
In the cis-isomer, the double bond holds the two carboxylic acid groups close together and in the same plane, whereas in the trans-isomer they are far apart. Heating the two isomers separately has very different effects.
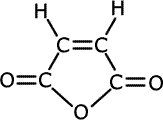
The cis-isomer easily loses water (dehydrates) to give a species known as an anhydride, specifically butenedioic anhydride.
 |
140ºC
moderate heat |
 |
|
cis-butenedioic acid
|
butenedioic anhydride
|
For this reaction to occur, the two carboxylic acid groups must be close together. They are not close enough in the trans-isomer, so the reaction cannot happen.
Nitrogen compounds
Nitrogen also exhibits geometric isomerism in compounds in which the nitrogen atom is double bonded. In this case the lone pair on the terminal nitrogen can be considered an attached group.
|
Example: The compound H-N=N-H has two geometric isomers. In one of the isomers the two hydrogens attached to the two nitrogens are on the same side of the double bond. This is the cis-isomer. In the other geometric isomer the two hydrogens 'cross' the double bond; the trans-isomer. |
The Cahn-Ingold-Prelog system of priorities
This assigns the attachment to the double bond a priority based on the atomic masses of the attached atoms. If they are the same then the next atom is examined.
When the highest priority groups are on the same side of the double bond the isomer is assigned the letter "Z", which comes from the German "Zusammen" meaning together. An easy way to remember is that Z isomers have the highest priority groups on the Zame Zide.
When the highest priority groups are on opposite sides of the double bond the isomer is assigned the letter "E" from the German "Entgegen" meaning against or towards.
 |
 |
|
Z-butenedioic acid
|
E-butenedioic acid
|
The advantage of the CIP system is that is allows treatment of stereoisomers that have four different groups attached to the double bond.

