|
The hydrocarbons are a massively important group of organic compounds as they constitute the majority of crude oil and natural gas. Hence they are the primary feedstock for the petrochemicals industry. |
|
Hydrocarbons
Hydrocarbons are compounds containing only hydrogen and carbon.
There is a huge diversity in the number of compounds that can be formed from only carbon and hydrogen atoms. They may consist of straight chains, branched chains or ring structures; they may contain only single bonds, double bonds, triple bonds, or aromatic systems in which electrons are delocalised over several carbon atoms.
To make sense of this diversity, hydrocarbons can be categorised into three main groups:
- Aliphatic hydrocarbons - straight and branched molecules
- Alicyclic hydrocarbons - ring structures
- Aromatic hydrocarbons - benzene and other delocalised ring structures
These names do not need to be learned, they are for reference only.
Sources of hydrocarbons
Hydrocarbons are mainly derived from fractional distillation and processing of crude oil, other fossil fuel or biotechnological sources. They are used as an energy source and a petrochemical feedstock (raw materials needed to produce other important chemicals and other materials such as plastics and polymers)
All hydrocarbons are flammable and produce carbon dioxide and water when burned in excess air or oxygen. Incomplete combustion may produce carbon monoxide and carbon microparticulates (small carbon containing particles). These products of incomplete combustion are a pollution hazard.
All complete combustion reactions can be represented by a general equation:
![]()
This allows investigators to find the formula of any organic hydrocarbon by measuring the carbon dioxide and water it produces on complete combustion.
Aliphatic hydrocarbons
Aliphatic hydrocarbons do not contain delocalised electron systems (benzene rings, primarily). They can be sub-divided into straight chains, branched chains.
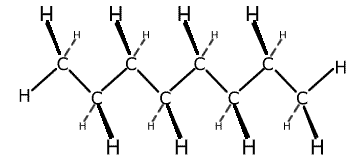
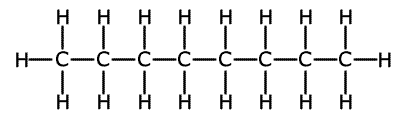
Straight chain hydrocarbons are actually a zig-zag shape, as the bond angle formed between carbon-carbon-carbon atoms is actually 109.5º. However, it is more convenient to represent the structure as though it were straight with right angles. The term 'straight chain' is used to differentiate the structures from branched chain molecules.
This is shown below using the straight chain molecule octane, C8H18.
|
Octane representations
|
|
 |
 |
|
3-dimensional representation
|
2-dimensional representation
|
Although the 2-dimensional (flat) structure is not strictly speaking correct, it contains all of the information that is needed.
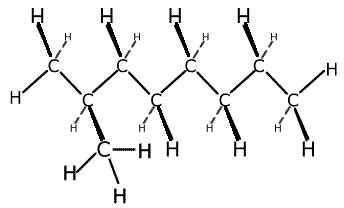
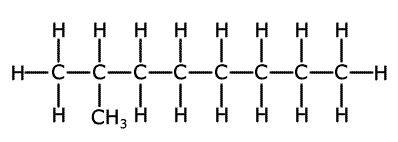
Branched chains have carbon containing chains branching off the main chain, just like branches of the main trunk of a tree. Once again, in reality the carbon atoms are all arranged in a zig-zag fashion.
|
2-methyloctane showing the branched
structure
|
|
 |
 |
|
3-dimensional representation
|
2-dimensional representation
|
Alicyclic hydrocarbons
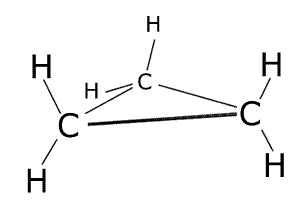
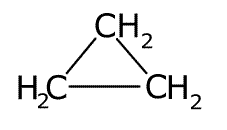
These are molecules that are arranged into ring structures. The prefix cyclo- is used to indicate the ring. The smallest of the hydrocarbon ring structures is cyclopropane, C3H6.
Small cyclic molecules have different chemical properties from their straight chain counterparts. Due to the higher energy of a strained ring structure they are more reactive. The impossibility of rotation about carbon-carbon bonds means that they display geometric isomerism when two or more other atoms or groups are bonded to the ring structure.
|
cyclopropane - C3H6
|
|
 |
 |
|
3-dimensional representation
|
2-dimensional representation
|
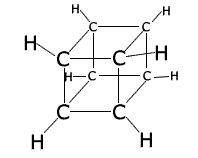
Ring structures can get very complicated, with one molecule having several different rings, for example, cubane C8H8.
Unsaturated hydrocarbons
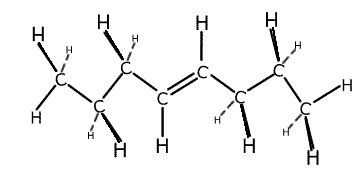
The terms saturation and unsaturation refer to the type of bonding between the carbon atoms in a structure. A molecule that contains only carbon-carbon single bonds is said to be saturated. Double bonds and triple bonds are said to be unsaturated.
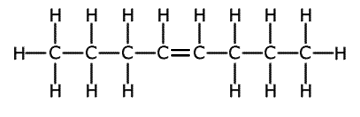
|
oct-3-ene
- C8H16
|
|
 |
 |
|
3-dimensional representation
|
2-dimensional representation
|
When a molecule contains many double (or triple) bonds it is said to be polyunsaturated. In long chain hydrocarbons unsaturation gives rise to 'kinks' in the structure and prevents the long chains aligning easily. This lowers the melting point.
The degree of unsaturation can be determined using reagents that react quantitatively with the double bonds.
Aromatic hydrocarbons
The actual definition of aromatic not necessary at this level, but they can be considered to be compounds that contain benzene rings. These have their own very characteristic properties.