|
Cell potentials refer to the voltage that is created between two half cells when they are connected via an external circuit.
|
|
Cell potential
When two half cells are combined using an external circuit and salt bridge, a potential difference arises due to the different electrode potentials of the two half cells.
The cell with the more negative potential will attempt to push electrons around the external circuit towards the less negative half cell. The measured potential (using a high resistance voltmeter) is called the cell potential - it is equal to the DIFFERENCE between the electrode potentials of the two half cells.
The direction of (attempted) flow is from the cell with the most negative potential to the cell with the least negative (most positive) potential.
|
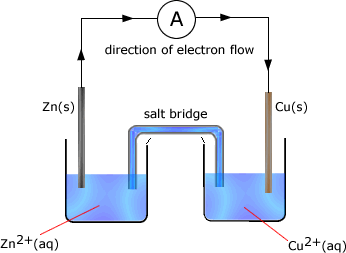
Example: In the following cell made up of two half cells the standard electrode potential of zinc = -0.76V and that of copper is +0.34V
The cell potential is the difference between the standard electrode potential values = -0.76 - +0.34 = -1.10V The zinc half cell has the most negative potential and so the direction of electron flow would be from the zinc half cell to the copper half cell. Reactions occuring in the half cells As the zinc half-cell releases electrons then..
As the copper half-cell accepts electrons then..
Adding these two reactions gives the overall cell reaction as:
cell potential = 1.10 V |
 Cu
Cu 
