|
Catalysts are substances of great importance in industry as they allow production of chemical substances to take place at faster more economical rates. |
|
Catalysts
Catalysts speed up reactions. They do so, by providing an alternative mechanism (route) for the reaction to proceed.
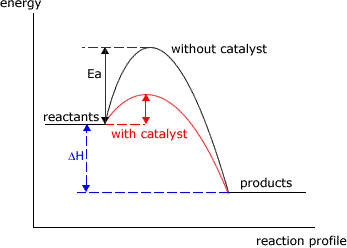
Equilibria consist of two opposing reactions, the forward reaction and the reverse reaction. A catalyst speeds up both of these reactions by an equal amount.
Consequently there is no change in the reactant and product proportions at equilibrium, when the reaction is catalysed. However, the reaction will achieve equilibrium proportions faster. For this reason catalysts are added to many industrial processes, even though they are equilibrium reactions.
|
Example: The reaction between sulfur(IV) oxide and oxygen to prepare sulfur(VI) oxide is an equilibrium as shown below: 2SO2(g) + O2(g) To reach equilibrium more rapidly, a V2O5 catalyst is added. This is known as the Contact process stage in the industrial manufacture of sulfuric acid. (see next section) |
Summary
1 Catalysts DO NOT affect the proportions of reactants and products at equilibrium
2 Catalysts DO NOT affect the value of Kc
3 Catalysts DO increase the rate of attainment of equilibrium.

