|
Many chemical reactions are reversible and never go to completion. For a system in equilibrium the rate of the forward reaction equals the rate of the reverse reaction and the concentrations of all reactants and products remain constant. |
Reversible reactions
These are reactions that can go in either direction.
| Reactants Products |
Although they might not seem to be the norm, they are in fact very common. Simply changing the conditions changes which direction in the reaction proceeds.
|
Example: The decomposition of copper(II) sulfate pentahydrate. The compound is a blue crystalline solid that loses water of crystallisation on heating according to the equation:
The product is a white powder, anhydrous copper(II) sulfate NOTE Although the term 'anhydrous' is used, there is still one water of crystallisation that is very hard to remove. If water is added dropwise to the product, there is an exothermic reaction and blue crystals are produced. The reaction has been reversed.
The first process requires energy and the second reaction releases energy. The reaction is reversible and could be written:
|
Dynamic equilibrium
If the reversible reaction can proceed in both directions at the same time, a situation may develop in which the reactants and products concentrations become constant, although both the forward and reverse reactions continue as before.
This is called dynamic equilibrium.
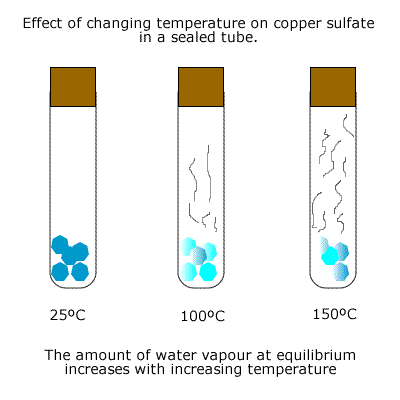
In the previous example of hydrated copper(II) sulfate, if the heating were carried out in a sealed tube then the water vapour produced in the reaction would create a reversible system, as it would tend to react with the anhydrous copper sulfate produced.
The extent of the equilbrium depends on the temperature used.