|
Human beings have colour vision, meaning that we can distinguish between the wavelengths from 400 to 700 nm, the most intense region of our sun's radiation that reaches the surface of the earth. Colour may be produced by emission of a source within this waveband or by absorption of wavelengths from a white light source. Transition metal complex solutions absorb a portion of the white light source leaving the transmitted light observable as coloured. |
|
Colour
Colour in materials or compounds is caused when the light reaching the eyes has some of the wavelengths removed by absorption. When we see an object as red it is only this colour that is reflected from the object while the other colours or wavelengths are absorbed.
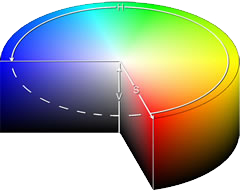
A coloured solution is caused by the white light passing through it and losing some of its wavelengths by absorption. If the solution appears blue it means that the complementary colours are absorbed by the solution.
In the complementary colour cylinder at the left, the colour that is opposite is the colour that is seen when one is absorbed.
For example, absorption of red light would leave the turquoise (cyan) colour showing. (image: wikipedia)
Coloured compounds
If colour is caused by the absorption of certain wavelengths from white light, the question remains - how are these wavelengths absorbed?
The colour in the transition metals (d-block) is usually due to the 'splitting' of the 'd' shell orbitals into slightly different energy levels. As a result, certain wavelengths of energy can be absorbed by the d-block elements (with electrons jumping between these slightly different energy levels), resulting in the complementary colour being observed.
The theory that explains the production of colour in this way is called crystal, or ligand field, theory.
This is not the only mechanism by which colour can be produced in compounds; there is also charge transfer and conjugation. Charge transfer involves the transfer of electrons from ligands to the transition metal orbitals and often produces intense colours.
One example is the deep purple colour of the manganate(VII) ion.
Conjugation is alternate double and single bonds in (usually) organic molecules, producing delocalised orbitals whose electrons can absorb light at visible wavelengths.
The azo-dyes are a good example, as are chromophores such as chlorophyll.
Crystal Field Theory
When ligands bond to transition metals they do so by donating electrons into empty hybridised orbitals.
The inner 3d orbitals are not involved in the bonding. They are, however, affected by the repulsive force of the donated electron pairs and the transition metal orientates itself to minimise the repulsions between the 3d orbital electrons and the ligands electron pairs.
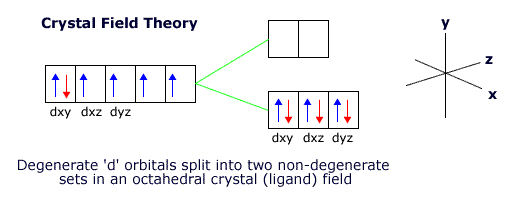
When the ligands are arranged octahedrally around the transition metal ion the lowest energy orientation for the ion involves keeping the dxy, dyz and dxz orbitals in the gaps between the ligands.
However, this puts the other two orbitals, the dx2-y2 and the dz2 orbital close to the ligands, raising their energy with respect to the other three orbitals.
The 3d orbitals are now no longer degenerate. This is called crystal field, or ligand field, splitting.
 |
If the crystal field (the electrostatic repulsion) is strong, then the '3d' orbitals are split further with more energy between the two different sets. This depends on the ligands.
Ligands can be arranged according to the strength of their electrostatic effect into an spectrochemical series.
Factors affecting colour
Anything that can affect the electrostatic field around the transition metal ion can affect the wavelengths of light absorbed and hence the colour transmitted by a solution, or reflected by a solid.
Colour in transition metal complexes is affected by three factors:
- 1 the transition metal
- 2 the oxidation state of the transition metal
- 3 the type of ligand
The transition metals have certain colours, or colour ranges that are typical of that metal.
Copper salts, for example, are usually blue or green, iron has salts that are pale green, yellow or orange.
The oxidation state is important.
Copper(II) salts are coloured, whereas copper(I) salts are white solids.
The reason for this lies in the electronic configurations of the two oxidation states.
- copper(II) [Ar] 4s0 3d9
- copper(I) 4s0 3d10
Copper(I) ions cannot absorb energy for d-d transitions as there are no empty, or partially empty, orbitals available to accept a promoted electron.
Table of some common transition metal complex ions and their colours
| Complex ion |
Oxidation state of metal
|
colour | ligand |
| [Fe(H2O)6]3+ |
+3
|
pale green | water |
| [Fe(H2O)6]2+ |
+2
|
yellow | water |
| [Cu(H2O)6]2+ |
+2
|
blue | water |
| [Cu(NH3)4]2+ |
+2
|
deep blue | ammonia |
| [CuCl4]2- |
+2
|
green | chloride ion |
The ligand can affect the colour in two ways.
Ligands all have different crystal field strengths and will split the 'd' orbitals by differing amounts. However, apart from this, the actual shape of the complex is defined by the type of ligand and the oxidation state of the transition metal.
If the shape of the complex changes, then this also causes a change in the type of crystal field splitting. Tetrahedral fields split the 3d orbitals in a different manner to an octahedral field.
|
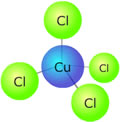
Example: Hexaaqua copper(II) ions are light blue in solution. The complex is octahedral, and there are six water molecules coordinated to the copper ion. If concentrated hydrochloric acid is added to the solution, the water ligands get replaced by chloride ligands and a new complex, tetrachlorocuprate(II) is formed. [Cu(H2O)6]2+(aq) + 4Cl-(aq)
This new complex has a tetrahedral geometry and a different crystal field splitting pattern. Its colour is now a deep green as the new energy difference between the non-degenerate '3d' orbitals absorbs light of a different wavelength. If concentrated ammonia solution is now added to the tetrachlorocuprate(II) solution, the colour fades and a light blue precipitate is formed, which then dissolves to form a deep blue solution. This final solution contains the tetrammine copper(II) complex, which is square planar. [CuCl4]2-(aq) + 4NH3(aq)
|

6-120.gif)
4-120.gif)
