|
Entropy has been called 'nature's arrow', as all natural systems move towards a state of maximum disorder. This is one of the statements of the second law of thermodynamics. The reasons for this lie in the statistics of natural systems, but for simplicity we can consider entropy to be the degree of disorder within a substance. Entropy is a state function, i.e. it depends only on the state of the substance and not how that state was reached. |
|
Disorder
 Disorder
is the natural state of things, just think about your bedroom! Without careful
attention a bedroom soon becomes cluttered with things all over the place.
Disorder
is the natural state of things, just think about your bedroom! Without careful
attention a bedroom soon becomes cluttered with things all over the place.
The reason for this is fairly logical; there is only one situation in which everything is ordered in a bedroom, all of the socks in the correct drawer, the bed made, the books on the shelves, tee-shirts folded etc etc. However, there are millions of ways in which the room can be untidy.
 If
nature is left to itself, the most likely situation is one of disorder - the
probability of disorder is much greater.
If
nature is left to itself, the most likely situation is one of disorder - the
probability of disorder is much greater.
Natural systems do not usually have someone to arrange them, they adopt the most likely arrangement.
Within a natural system there are billions upon billions of particles, each of which can be given a wide range of energies. The system adopts one of the most likely states, i.e. a disordered state.
Entropy
Entropy is the term given to the natural disorder of the universe. If left to itself, the universe tends towards disorder. It can be thought of as an inevitable driving force. Entropy has been called 'nature's arrow'. It is the natural tendency of systems to become disordered.
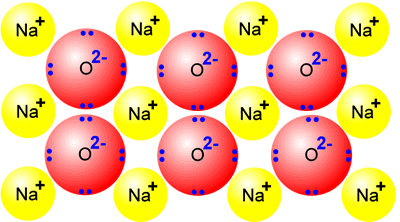 If
we look at a solid, we can see that all of the particles are carefully arranged
in specific locations. This is a highly organised and ordered system. Its
entropy is said to be very low.
If
we look at a solid, we can see that all of the particles are carefully arranged
in specific locations. This is a highly organised and ordered system. Its
entropy is said to be very low.
However, in a gas the particles are free to move randomly and with a range of speeds. The entropy of gases is high.
A liquid has more entropy than a crystalline solid, but much less than a gas.
A solution contains a mixture of solute and solvent particles and has more entropy than a simple liquid but, once again, far less than a gas.
|
State
|
Entropy
|
|
solid
|
very low
|
|
liquid
|
low
|
|
gas
|
high
|
Factors affecting entropy
