|
Hess' law may also be applied to dissolution of ionic compounds. |
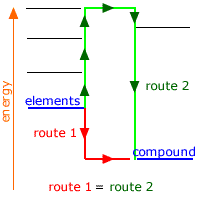
Born-Haber Cycle
|
Definition of solution enthalpy
The energy change when 1 mole of a substance is dissolved in a solution to infinite dilution.
MgSO4(s) ![]() Mg2+(aq) + SO42-(aq)
Mg2+(aq) + SO42-(aq)
Clearly, the idea of infinite dilution is not to be taken literally. It just means that a solution is prepared by dissolving the solute to such a dilution as would produce no further energy change.
Dissolving ionic compounds
When an ionic compound dissolves the ions in the giant lattice must separate and disperse into the solution. The large amount of lattice enthalpy needed to break the lattice is compensated by the hydration enthalpy of the ions and the increase in entropy in the disordered solution.
The actual energy change on dissolution is a balance between the individual energy steps involved in the process. It may be endothermic or exothermic overall.
The mechanism of dissolution
The basic process involves going from ions in fixed positions within a lattice to free solvated ions in solution. This can be broken down into specific energetic steps in a similar approach to that of the Born-Haber cycles.
These energetic steps are as follows:
- The lattice enthalpy of the ionic solid
- The hydration enthalpies of the ions
The enthalpy of solution is calculated from the sum of the steps involved.
Enthalpy of solution = lattice enthalpy (endothermic) + hydration enthalpies (exothermic)
Lattice enthalpy
The first energetic step is to turn the ionic lattice into gaseous ions at infinite separation. This is energetically equivalent to the lattice enthalpy. As energy is needed to separate the ions this step is endothermic.
NaCl(s) ![]() Na+(g) + Cl-(g)
Na+(g) + Cl-(g)
The magnitude of the lattice enthalpy is determined by the charge on the ions and the radius of the ions, and to a lesser extent, by the actual structure of the crystal lattice.
Hydration enthalpy
This is defined as the enthalpy change when 1 mol of a gaseous ion is solvated by solvent molecules. It may be represented by the equation:
X-(g) + (aq) ![]() X-(aq)
X-(aq)
The hydration enthalpy is exothermic as bonds are formed between the dissolving ion and the solvent molecules. The strength of the bonding depends on the charge on the ion and its ionic radius.
The most important factor is the magnitude of the charge:
- High charge magnitude = stronger bonds = greater (exothermic) energy change
- Small ions = strong bond = larger (exothermic) energy change
The term "charge density" is occasionally used to refer to the charge/volume ratio of an ion. High charge density is associated with strong bonds.
The enthalpy of solution - experimental
The procedure simply involves measuring the temperature change in a known mass of solvent when know number of moles of solute is dissolved.
|
Example: 10g of ammonium nitrate (NH4NO3) is stirred into 100cm3 of water and the temperature change recorded. If the temperature decreased by 7ºC what is the enthalpy of solution of the ammonium nitrate. (specific heat capacity of the solution = 4.2 kJ kg-1 ºC-1) Energy change in the water = mass x specific heat capacity x temperature change Energy change = 0.1 x 4.2 x 7 Energy change = +2.94 kJ (the sign is positive because the water temperature decreases, i.e. heat is being transferred from the water to the ammonium nitrate - it is an endothermic change) Moles of ammonium nitrate = 10/80 = 0.125 moles ∴ enthalpy of solution of ammonium nitrate = 2.94/0.125 = +23.52 kJ mol-1 |
Application of Hess' law
Determination of solution enthalpy is a simple matter experimentally and if the lattice enthalpy of the compound is known, a Hess' cycle allows us to find the hydration enthalpies of the ions.