| In common with many theoretically calculated values, reaction enthalpies often differ from values found experimentally. In this section we examine the reasons behind experimental deviation. |
|
Calculated reaction enthalpy values
Values of enthalpy calcuated using the bond enthalpy terms are often far from the values found experimentally. This is due to the fact that they are average values and do not take into account situations in which the bond strength is different from the average.
Possible causes of variation include:
- The inductive effect
- Steric factors
- Delocalisation
Bond energies are measured over a range of different gaseous molecules and do not take into account any inter- or intramolecular forces that may influence the actual bond energy value of the molecule under consideration.
The inductive effect
Bonds may be weakened by the inductive effect of other atoms in close proximity within the molecule. Highly electronegative atoms tend to draw electrons towards themselves along the bonds and this influence may be felt at several bonds distance.
For example, the effect of a chlorine atoms in trichloroethanoic acid cause electrons to be withdrawn from the O-H bond of the carboxylic acid group, making it easier to be broken. Consequently trichloroethanoic acid is a stronger acid than ethanoic acid.
Steric factors
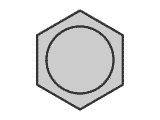
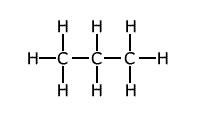
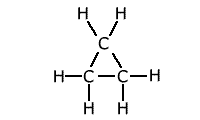
The presence of strain in a molecule due to the orientation of bonds being forced into a configuration that is not natural, causes weakening of the bonds. A good example of this is the bond strength of small cyclic molecules, such as cyclopropane, when compared to normal propane. The C-C bond strength is much lower in cyclopropane than in propane, due to the fact that the bond angle is constrained at 60º, instead of the 109º 28' of the usual tetrahedral carbon bond angle.
|
Example: C-C bond strength in propane and cyclopropane
|
This extra 'ring strain' weakens the carbon -carbon bonds considerably. Steric effects may also appear in non-cyclic systems, in which the presence of 'bulky' groups causes strain within the molecule.
Delocalisation
Delocalisation occurs whenever there are molecular orbitals spread over more than two charge centres (atoms).
 This
may arise due to conjugation, i.e. alternating double and single bonds within
a molecule, or in systems where lone pairs can become involved in the bonding,
for example the carboxyate group.
This
may arise due to conjugation, i.e. alternating double and single bonds within
a molecule, or in systems where lone pairs can become involved in the bonding,
for example the carboxyate group.
Delocalisation due to conjugation
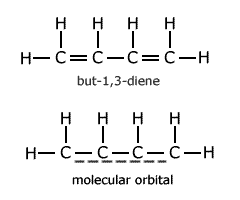
One of the simplest cases is that of but-1,3-diene. The molecule is usually drawn showing alternate double and single bonds.
However, evidence suggests that there is a molecular orbital spread over all four carbon atoms. This means that reaction enthalpy calculations, using C-C and C=C bond enthalpies, cannot be applied for reactions involving but-1,3-diene. The actual bond enthalpy of the C-C bond lies somewhere between that of a C-C single bond and a C=C double bond.
The benzene ring
The most well-known situation in which the bonding is delocalised over a complete molecule is that of benzene, C6H6. The classical structure of benzene shows alternating double and single bonds arranged in a hexagonal ring of carbon atoms.
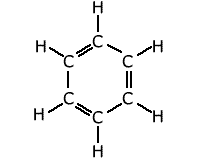 However,
measurements of bond lengths and bond strengths show that all of the carbon-carbon
bonds are identical. There is a delocalised molecular orbital spread over
the whole ring of carbons giving each C-C bond a character between that of
a double and single bond.
However,
measurements of bond lengths and bond strengths show that all of the carbon-carbon
bonds are identical. There is a delocalised molecular orbital spread over
the whole ring of carbons giving each C-C bond a character between that of
a double and single bond.
For example: the C-C single bond length = 0.159 nm, C=C double bond = 0.134 nm, while the C-C bond length in benzene is 0.139 nm.
Clearly, calculations of reaction enthalpies using average bond energy terms cannot be applied accurately to molecules containing benzene rings.
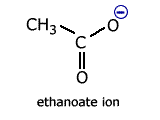
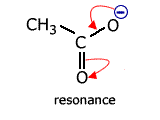
Delocalisation involving heteroatoms - the carboxylate ion
Carboxylate ions, for example the ethanoate ion, have two carbon-oxygen bonds.
 |
 |
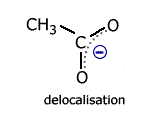 |
In the classical structure, these appear to be of different length.
However, once again there is a delocalised molecular orbital spread over three charge centres (atoms). This has the effect of making the two carbon - oxygen bonds identical and, consequently, any calculation of reaction enthalpy using carbon-oxygen single and carbon = oxygen double bonds is inaccurate.