| Chemical reactions produce new substances which nearly always have a different chemical energy to the reactants. This section looks at how these reactions are categorised into exothermic and endothermic. |
Chemical reactions
Chemical reactions involve the creation of new substances. These new substances have different chemical energy to the original reactants. This means that energy may be either released, or absorbed, in the course of a chemical reaction. Any chemical energy change produces a corresponding thermal energy change as the chemical energy is converted into thermal energy.
The change in chemical energy of a system is called the enthalpy change. It is represented by the symbol Δ H.
There are two possibilities
- 1 Chemical energy
 Heat energy (the temperature of the system increases)
Heat energy (the temperature of the system increases) - 2 Heat energy
 Chemical energy (the temperature of the system goes down)
Chemical energy (the temperature of the system goes down)
In the first instance there is an increase in thermal energy - this is called an exothermic reaction and in the second case there is a decrease in thermal energy. This is called an endothermic reaction.
Exothermic reactions
| Chemical energy |
These are the norm in chemical reactions.
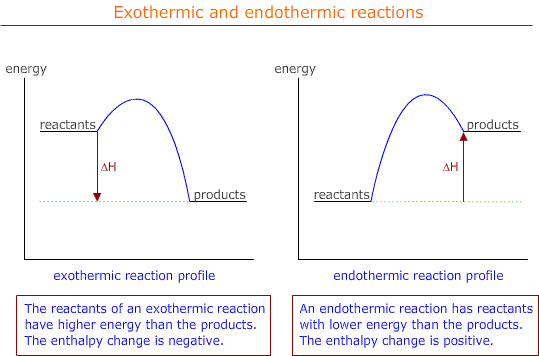
As the process occurs, the temperature of the system increases and the chemical energy goes down. This can be displayed on an energy profile as shown below
By convention the enthalpy change is assigned a negative value. ΔH = negative
Endothermic reactions
Endothermic reactions proceed with a increase in chemical energy from reactants to products.
| Heat energy |
The system changes some of its thermal energy into chemical energy and loses temperature in the process.
By convention, the enthalpy change is assigned a positive value. ΔH = positive.