|
The concept of heat and temperature are often not clear to students. It is important to understand these concepts on a molecular level before proceding to use them. |
The energy concept
It is an observation that when substaces are heated their temperature increases. Although this may seem like an obvious statement, it is important to understand the processes that are involved. To do this we have to invoke the concepts of energy and temperature.
Energy is a concept that is very difficult to define without using examples. Certain forms of energy are easy to understand due to common experience, such as kinetic energy.
We appreciate that a moving body possesses 'kinetic energy' and that if something gets in its way then there will be collision and an effect (breakage, noise, deformation etc).
As the kinetic theory tells us that all particles are in motion then we can refer kinetic energy to this motion and say that the particles have energy due to their movement. The total energy is the product of the average kinetic energy of a particle multiplied by the number of particles.
However, particles are not only in translational motion, they are also capable of vibrations and rotations. The total amount of motional energy they possess is a combination of these.
When a substance is given energy in the form of heat, the particles of the material respond by increasing their kinetic energy. The vibrations, rotations and translations of the particles in the material increase. We say that the substance has become 'hotter', although in fact all that has happened is that the particles are now moving faster.
In order to quantify this increase in particular kinetic energy, we must have a means of measuring and from here arises the idea of temperature scales and thermometers.
Temperature
Temperature is a measure the average heat energy content of the system under study.
The concept of 'heat' is easy to understand, as we have senses capable of detecting changes in heat. We say that something is warm or cold. However, the sensation of heat is just that, a sensation, something interpreted by the brain and understood by experience. What is actually occurring is a transfer of vibrations from the heat source to the sensors in the skin. These sensors produce an electrical signal that travels through nerve fibres to the brain, where we experience the sensation.
Our idea of 'heat' is simply the degree of vibrations experienced by the sensory cells. Clearly, this is OK for biological necessities, but hardly empirical. Scientists soon realised the need of enumerating this hot/cold reflex, and invented the thermometer.
Recognising that certain materials respond to heat/cold by expansion/contraction, it was possible to enclose a sample of a suitable material into a glass tube and to watch it expand or contract as the local environment temperature changed. A scale was needed that was common experience for people all over the world. The two key reference points on the scale chosen were the freezing point (melting point) and boiling point of water. A scale was constructed between these two points and subdivided into 100 units (the Celsius scale). Nowadays the Celsius scale is the scale of choice for most purposes.
The thermometer responds to the average motion of the particles in the environment being measured. It should be recognised that when the thermometer registers 80ºC, it is informing us that the motion of the particles in the substance being measured has caused motion of the particles in the mercury, resulting in the mercury in the thermometer expanding to the 80º mark on the Celsius scale.
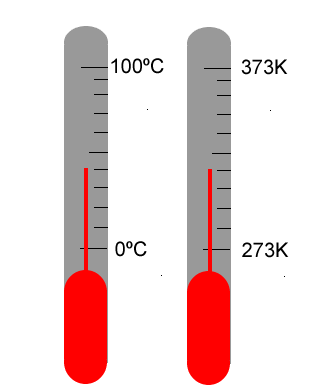
The Celsius scale is a relative scale, relative to the melting and boiling points of water. A more scientific scale is the absolute scale of temperature measurement in which the magnitude of a degree is the same as 1 degree Celsius, but the zero of the scale starts at absolute zero. This is also called the Kelvin scale; the unit of measurement is the Kelvin, K.
|
273 Kelvin = 0º Celsius 373 Kelvin = 100º Celsius |
Absolute zero is the temperature at which there is no particle motion whatsoever. It is equal to -273.16 ºC under current definitions.
Heat energy
The total energy of a sample is a function of both the temperature and the mass of particles present. The temperature gives a measure of the average kinetic energy of the particles in the system.
|
Example: Which contains the most energy, a bathful of lukewarm water or a spark at 2000ºC? A spark may have a temperature of 2000ºC, but it contains less energy than a bathful of lukewarm water. |
In reality, it is difficult to measure the absolute quantity of kinetic energy contained within a sample and it is of limited value. We are more concerned with changes in the heat energy, as this also reflects changes in the chemical energy of the system, as we shall see in the next section.
|
It is important to remember that all substances at
the same temperature have the same average kinetic energy.
|

