|
The term 'lattice' means a regular array or network of something. |
|
An ionic lattice is a regular repeating arrangement of ions in three dimensions. The ions ocupy files and rows and columns. Each negative ion is surrounded by positive ions and each positive ion is surrounded by negative ions. The actual packing structure of the ions depends on the relative sizes of the ions and their respective charges.
The lattice strength depends on the forces of attraction between the positive and negative ions. This electrostatic force depends on two factors:
- The magnitude of the charge on the ions
- The distance between the ions (the sum of the ionic radii)
The equation for determining the electrostatic force of attraction is: ![]()
In this equation the term ![]() is just a series of constants and can be combined together to give:
is just a series of constants and can be combined together to give: ![]()
In summary, the force of attraction between two ions is proportional to the product of their magnitudes and inversely proportional to the square of the distance between them.
The strength of a lattice is a function of its lattice enthalpy, the energy required to overcome the forces of attraction keeping the ions togerther in the lattice. This is defined as the energy required to separate one mole of a crystal lattice into gaseous ions at infinite separation. For example sodium chloride lattice enthalpy is given by:
NaCl(s) ![]() Na+(g)
+ Cl-(g) ΔH = +790 kJ mol-1
Na+(g)
+ Cl-(g) ΔH = +790 kJ mol-1
Ions with a double charge produce lattices with a much higher lattice enthalpy.
|
Example: Compare the magnitude of electrostatic force in the lattices MgO and NaCl, given the following information:
In the MgO lattice both of the ions have a double charge so the product of the charges is for times as great as for NaCl. The sum of ionic radii for MgO = 0.205nm and for NaCl is 0.279nm. Squaring both terms we get: for MgO: 0.2052 = 0.0420, for NaCl: 0.2792 = 0.078. Therefore the r2 term for NaCl is aproximately twice as large as that of MgO. We would expect this to have the effect of doubling the electrostatic force. So the electrostatic force in the MgO lattice is has a numerator four times greater than NaCl and a denominator half as large as NaCl. Clearly the force of electrrostatic attraction in MgO is roughly eight times greater than in NaCl. Note: You will not be expected to do a mathematical treatment of ionic force in the exams, but it is useful to compare the forces involved. |
Sodium chloride, NaCl, can be considered a typical ionic compound. The structure often appears in examinations and should be familiar to students. It can be used as an example of the bonding and structure of all of the compounds formed between group 1 and group 17 elements.
Perfect and imperfect lattices
In some substances the calculated lattice energies vary considerably from the experimentally determined values. This is usually because the lattice has a degree of covalent character. What does this mean?
An ionic substance is made by electron transfer from metal atoms to non-metal atoms creating ions. In a perfect ionic substance these ions are not affected by one another. However, when the negative ion is large and the positive ion is small (or/and has a high charge), the high charge density of the positive ion can polarise the electron charge cloud of the large negative ion, pulling electrons back towards itself. This gives a distorted ionic charge field, or to put it another way, a degree of covalent character.
 |
 |
|
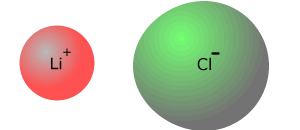
Lithium chloride is almost wholly
ionic
|
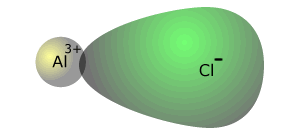
The high charge density aluminium
ion distorts the charge cloud on the chloride ion, making the bond covalent.
|
If the lattice has a degree of covalent character, it is tending towards simple molecular behaviour, in which the lattice is much weaker. The experimentally determined lattice energy is correspondingly smaller than the theoretically determined value.
All ionic compounds exist in a giant ionic lattice of repeating oppositely charged ions. The formula of the ionic compound is the simplest ratio between the ions within the lattice. For this reason the term "relative formula mass" is used when dealing with an ionic compound.
The strength of the ionic lattice is a function of both the charge on each ion and the radius of the ions.
- Greater charge = stronger lattice
- Smaller size = stronger lattice
This can be demonstrated by both the lattice enthalpy and the melting point of the lattice. The degree of ionic character is determined by the difference in electronegativity between the elements involved in the compound.
- Greater difference in electronegativity = more ionic character
Decreasing the degree of ionic character decreases the lattice strength and melting point.

