|
Vectors are quantities that have both magnitude and direction. They can add up, or cancel out, depending on orientation. Dipoles are vector quantities. |
|
Resolution of dipoles
Dipoles are similar to forces in that they can be resolved, added and cancelled out. If the dipoles within a molecule do not cancel out, then the molecule itself is polar, i.e. there is a region of positive charge within the molecule and another region of negative charge.
|
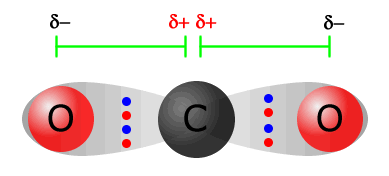
The simplest case is probably that of carbon dioxide
|
The carbon = oxygen bond is polarised due to the difference in electronegativity between carbon and oxygen (2.5 and 3.5 respectively). There is a horizontal dipole on the left of the molecule, but there is also another horizontal dipole with the same magnitude, but the opposite direction on the other side of the molecule. Both of these dipoles cancel out, leaving a molecule that is non-polar. |
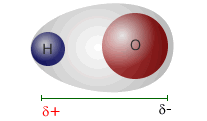
Water is a polar molecule in which the dipoles do not cancel out. This is because of the shape of the water molecule. Although the O-H bonds are both equally polar they are not exactly opposite one another and cannot cancel out. In fact they cancel out in the horizontal plane (as drawn), but not in the vertical.
|
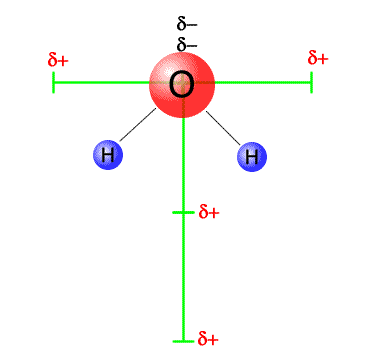
Vector dipoles in water
|
The dipoles in the water molecule can be resolved into horizontal and vertical components (green bars). The horizontal components are equal in magnitude, but opposite in direction and cancel out. The verticle dipoles are equal in magnitude and act in the same direction and so they add up. The molecule is polar overall, with a vertical dipole, negative at the top (near the oxygen) and positive at the bottom (between the two hydrogens) |
Polarity of molecules
The polarity of a molecule can be determined by firstly considering the relative electronegativities of the component atoms to find polarised bonds (dipoles), then inspecting the molecule to see if the individual dipoles cancel out. It is important to remember that the symmetry of a molecule is important in this respect.
- 1 Linear dipoles cancel out if the components are identical and oppositely arranged, such as in BeCl2 and CO2.
- 2 Trigonal planar molecules dipoles cancel out if all three atoms (or groups) bonded to the central atom is the same, such as BF3.
- 3 Tetrahedral molecules' dipoles cancel out if all four attached atoms (or groups) are the same, such as CCl4.
Consequences of polarity
Polar molecules are 'sticky' in that they are more attracted to one another. They behave as if they are tiny magnets, although the force between them is electrostatic, not magnetic.
The more polar they are the greater the degree of intermolecular force. This is covered in greater detail in subsequent sections.
The polarity of liquids can be easily demonstrated using a stream of liquid from a burette and approaching it with a charged electrostatic rod. The stream of liquid bends towards the rod if it is polar.