|
The mass spectrometer is an important analytical instrument in both inorganic and organic chemistry. Nowadays, it may be combined with an initial gas chromatography stage giving an instrument that both separates and analyses at the same time (GCMS). |
|
Instrumental details
A sample is injected into the mass spectrometer and is vaporised before meeting a stream of high energy electrons, that turn the atoms into ions (by dislodging electrons) or, if we are dealing with molecules, causes the molecules to break apart (fragment). The ions that are produced in each case are deflected by magnetic fields and detected with a high degree of accuracy.
The final read-out may be graphical or digital and gives information about the relative abundance of all of the ions produced by the stream of electrons, as well as their exact masses.
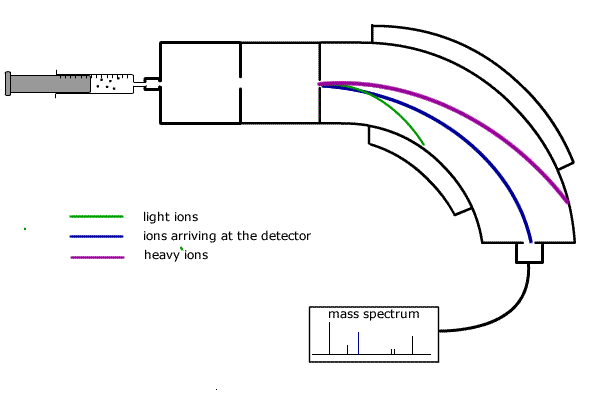
In summary a single beam mass spectrometer has the following stages of operation (refer to diagram below):
![]() Injection
-The sample is injected into the vaporisation chamber,either as a gas or in
an inert solution.
Injection
-The sample is injected into the vaporisation chamber,either as a gas or in
an inert solution.
![]() Vaporisation
- If necessary, the sample is heated to produce a vapour and the gas diffuses
into the...ionisation chamber.
Vaporisation
- If necessary, the sample is heated to produce a vapour and the gas diffuses
into the...ionisation chamber.
![]() Ionisation
- An electron beam knocks electrons off the vaporised particles, producing
positive ions.
Ionisation
- An electron beam knocks electrons off the vaporised particles, producing
positive ions.
![]() Acceleration
- The positive ions are attracted towards negatively charged plates.
Acceleration
- The positive ions are attracted towards negatively charged plates.
![]() Deflection
- The stream of ions passes through into the magnetic field which deflects
them into a curved path.
Deflection
- The stream of ions passes through into the magnetic field which deflects
them into a curved path.
![]() Detection
- The magnetic field is varied by the controller and ions with different masses
are directed into the detector.
Detection
- The magnetic field is varied by the controller and ions with different masses
are directed into the detector.
The operating principles are no longer a requirement for core and AHL.
Operating principles
The curved path travelled by each ion depends on its mass (actually the mass:charge ratio, but as the charge is always the same and equal to the charge on an electron, but positive, then we can talk about the mass alone). Heavier ions have more momentum and require more force for deflection.
The magnetic field strength of the deflector coils is varied and allows detection of all ions according to their mass to charge ratio.
The ions arriving at the detector constitute a flow of positive charge and can be recorded electronically.
In this way, the relative atomic mass of each 'peak' is recorded on the mass spectrum read-out. The height of the peak represents the abundance of the specific particle.
Fragmentation
The energy of the electrons in the ionisation chamber of the mass spectrometer not only can ionise the molecules it encounters, but also cause the ions produced to fragment into smaller pieces. This is called fragmentation. The smaller pieces, or fragments, themselves may be detected if they are in the form of ions. The peak that occurs at the highest m/e value is called the molecular ion. It is the ion produced by removing one electron from the molecule itself and can be used to determine the relative molecular mass of the species under investigation.
A typical molecular fragmentation pattern may look as follows:
|
Fragmentation of ethanol, CH3CH2OH 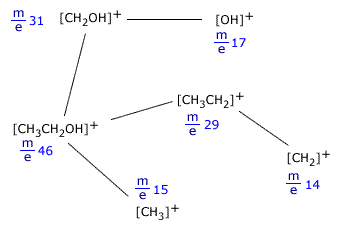
Notice that only ions are shown as only ions can be detected in the mass spectrometer. When an ion fragments, it makes neutral species as well, but these cannot be detected, as they can neither be accelerated nor deflected by the magnetic field. Example:[CH3CH2OH]+ In this fragmentation, [OH] is produced but does not cause a trace on the spectrum. |
The mass spectrum of chlorine Cl2
The chlorine spectrum shows several lines, all of which are due to positive ions formed in the mass spectrometer ioniser stage.
The line with the highest m/e value comes from the molecular ion, it is due to the [Cl2]+ ion. Chlorine has two different isotopes, therefore there are three possible molecular ions.
- [37Cl-37Cl]+
- [35Cl-37Cl]+
- [35Cl-35Cl]+
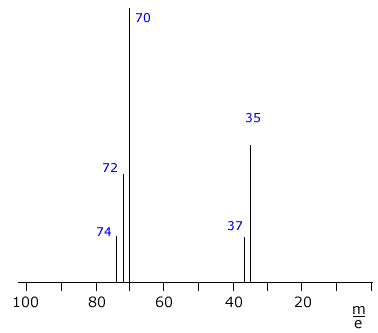 |
The remaining two lines at m/e 35 and m/e 37 are due to
the ions [35Cl]+ and [37Cl]+
formed by fragmentation of the molecules.
The heights of the lines can be used to calculate the isotopic abundances. In this case, the relative heights of the m/e 35 and m/e 37 lines is 3:1, showing the natural abundance of 35Cl and 37Cl to be 75% to 25% respectively. |

